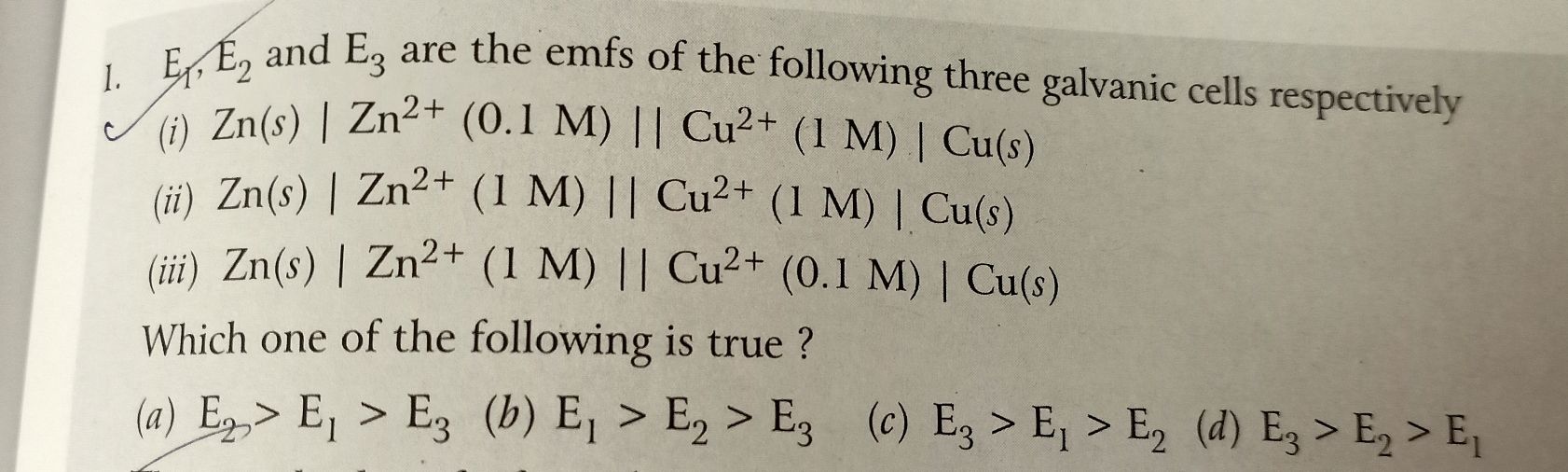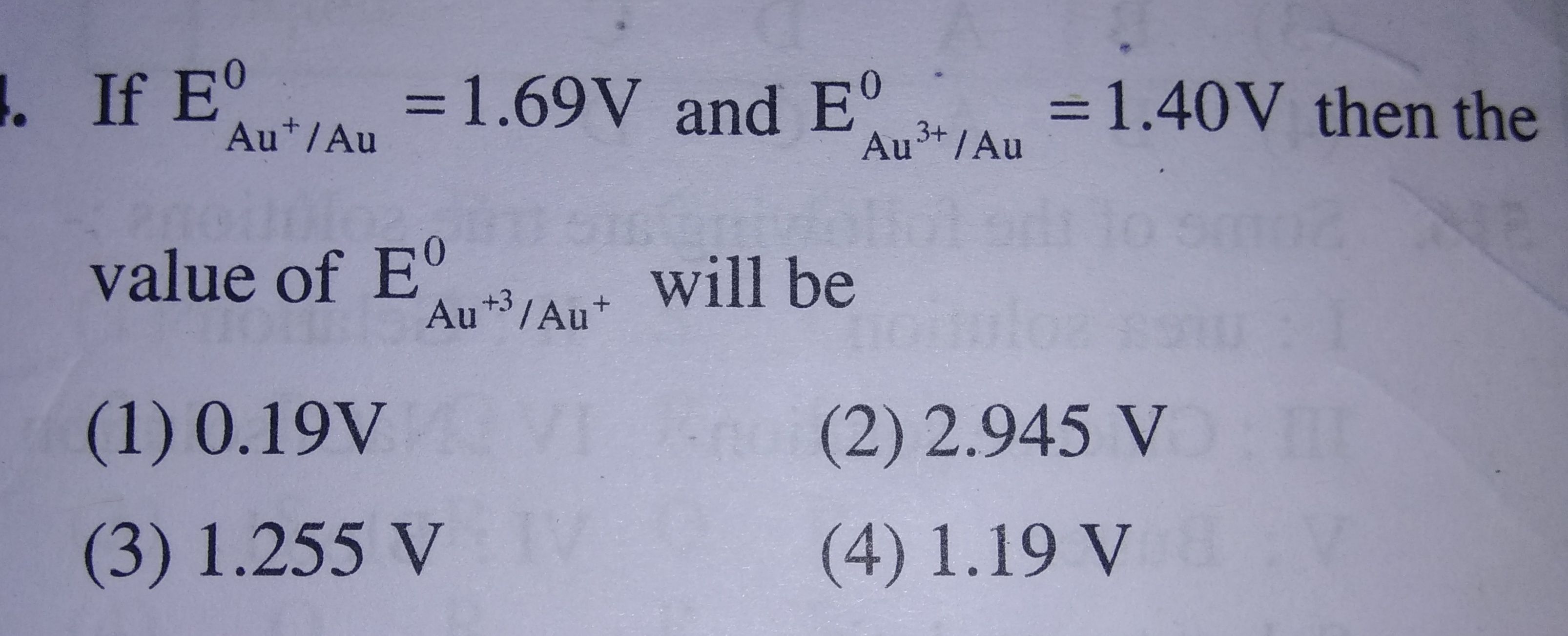CBSE Class 12-science Answered
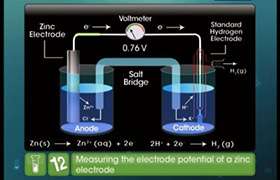
why does copper have positive electrode potential? it has same inthalpy of ionisation(2) as nickel(-2121) so it should also have a negative value
Asked by prakriti12oct | 11 Nov, 2019, 12:09: AM
Copper has lower tendency than hydrogen to form ions, so if the standard hydrogen electrode is cconnected to the copper half-cell, the copper will be relatively less negative. The copper electrode has relatively lower number of electrons. so it has positive electrode potential.
Answered by Ravi | 11 Nov, 2019, 03:11: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by rajpatil | 19 Jun, 2020, 07:12: PM
CBSE 12-science - Chemistry
Asked by piyushkhariyal | 06 Mar, 2020, 10:36: PM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 15 Feb, 2020, 08:21: PM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 11 Nov, 2019, 12:09: AM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 05 Nov, 2019, 04:50: PM
CBSE 12-science - Chemistry
Asked by Balbir | 21 Aug, 2019, 09:14: PM
CBSE 12-science - Chemistry
Asked by ankitathapliyal097 | 24 Jul, 2019, 10:05: PM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 26 Jun, 2019, 02:18: PM
CBSE 12-science - Chemistry
Asked by jhajuhi19 | 01 May, 2019, 09:03: PM
CBSE 12-science - Chemistry
Asked by pardeepkumar2281 | 26 Sep, 2018, 09:00: PM