CBSE Class 12-science - Standard Electrode Potential Videos
Standard Electrode Potential - Part 1
This video explains the construction of electrochemical cell, the concept of electrode potential and the calculation of cell potential by using electrode potential.
- What is a cell constant
- Electrochemical series are the series of element in which the elements are arranged in ........order of their reducing strength
- Why is the value of standard electrode potential of Mn, Co,Ni,Zn more negative than expected? Is the standard electrode potential more negative than expected for cobalt? (according to graph pg217 NCERT chemistry)
- why does copper have positive electrode potential? it has same inthalpy of ionisation(2) as nickel(-2121) so it should also have a negative value
- The discharge potential of Chloride ions is less than hydroxide ions . what does this line mean and why chloride has less discharge potential.
-
Please answer this question.
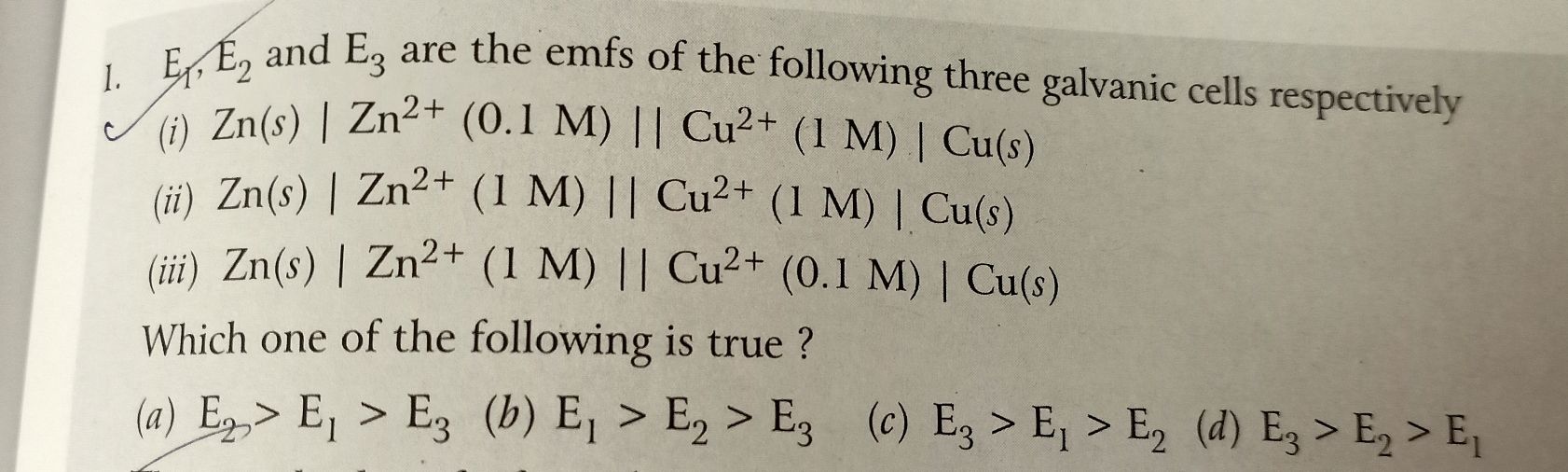
- A Cu rod is dipped in 0.1M CuSo4 solution.Calculate the potential of this half cell if CuSo4 undergoes 90% dissociation at 25 C.
- Please give the series of increasing ease of getting discharged at anode?
-
Plzzzzzz help me in this... (Refer to attachment)
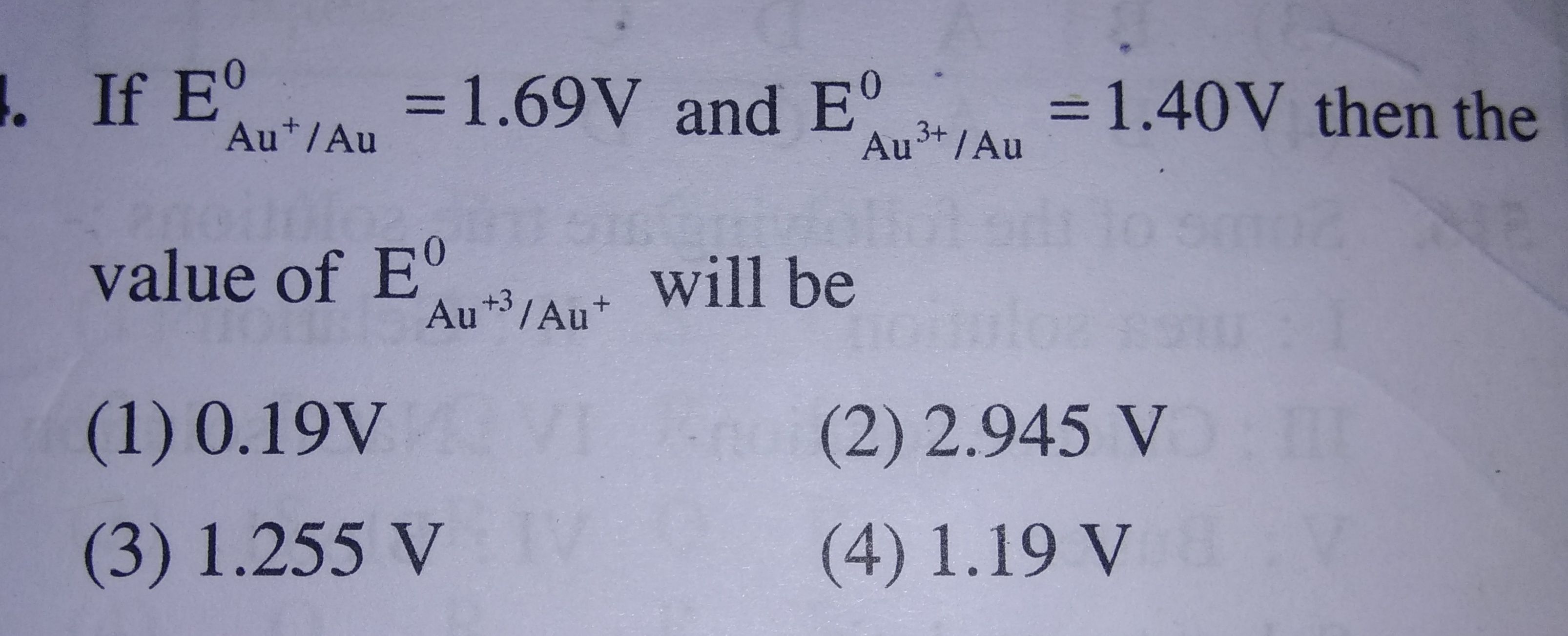
- CALCULATE POTENTIAL OF HYDROGEN ELECTRODE IN CONTACT WITH A SOLUTION OF PH 10

