CBSE Class 12-science Answered
There is also a significant difference between the solvents: CCl4 and H2O. The difference between the electro negativities of the carbon and chlorine atoms in CCl4 is so small (EN = 0.56) that there is relatively little ionic character in the C![]() Cl bonds.
Cl bonds.
Even if there was some separation of charge in these bonds, the CCl4 molecule wouldn't be polar, because it has a symmetrical shape in which the four chlorine atoms point toward the corners of a tetrahedron, as shown in the figure below. CCl4 is therefore best described as a nonpolar solvent.
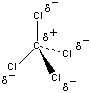
The difference between the electronegativities of the hydrogen and oxygen atoms in water is much larger (EN = 1.24), and the H![]() O bonds in this molecule are therefore polar. If the H2O molecule was linear, the polarity of the two O
O bonds in this molecule are therefore polar. If the H2O molecule was linear, the polarity of the two O![]() H bonds would cancel, and the molecule would have no net dipole moment. Water molecules are not linear, however, they have a bent, or angular shape. As a result, water molecules have distinct positive and negative poles, and water is a polar molecule, as shown in the figure below. Water is therefore classified as a polar solvent.
H bonds would cancel, and the molecule would have no net dipole moment. Water molecules are not linear, however, they have a bent, or angular shape. As a result, water molecules have distinct positive and negative poles, and water is a polar molecule, as shown in the figure below. Water is therefore classified as a polar solvent.
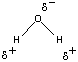
Because water molecules are bent, or angular, they have distinct negative and positive poles. H2O is therefore an example of a polar solvent
So because of their different nature the two do not dissolve with each other and on mixing them we see two separate layers of two solvents.








