CBSE Class 12-science Answered
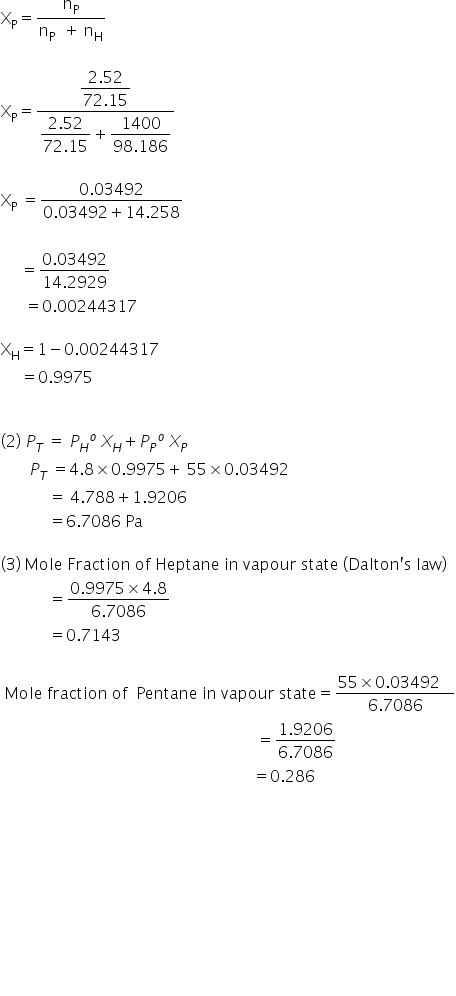
pentane and heptene have a vapour pressure of 55pa and 4.8 respectively at 20oc. A mixture is known to contahoeptin 2.52g of pentane and 1400g of heptene.calculate
a.the mole fraction of each components in the liquid
b.the total vapour pressure of the mixture.
c.the mole fraction of each component of the vapour phase
Asked by jenniferohwo | 03 Sep, 2019, 07:33: AM
(1) Mole Fraction of Heptane in liquid =XH
Mole Fraction of Pentane in liquid=XP
Answered by Ravi | 03 Sep, 2019, 17:45: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by manishachand010 | 27 May, 2022, 10:14: AM
CBSE 12-science - Chemistry
Asked by gangajyothibansode | 12 Aug, 2020, 06:08: AM
CBSE 12-science - Chemistry
Asked by mukeshtiwariji214 | 13 Jul, 2020, 07:42: AM
CBSE 12-science - Chemistry
Asked by rufinshafeek | 13 May, 2020, 14:54: PM
CBSE 12-science - Chemistry
Asked by unnisidharthpaleri | 13 May, 2020, 10:51: AM
CBSE 12-science - Chemistry
Asked by shrutikiran2000 | 05 May, 2020, 04:27: AM
CBSE 12-science - Chemistry
Asked by pampa7799 | 10 Feb, 2020, 07:45: AM
CBSE 12-science - Chemistry
Asked by jenniferohwo | 03 Sep, 2019, 07:33: AM
CBSE 12-science - Chemistry
Asked by riyap0429 | 07 Aug, 2019, 11:16: AM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 14 Apr, 2019, 18:13: PM








