CBSE Class 11-science Answered
Why 2nd electron gain enthalpy of oxygen is positive.if it is positive means we need to give energy to oxygen to put 2nd electron in it .but if we are providing energy the electron inside the oxygen should move to exited state means higher energy state.how we can say that it is positive?
Asked by Kamlesh Sahu | 27 Jul, 2014, 07:24: AM
When an electron is added to an isolated oxygen atom, it becomes uninegative ion. Now if one more electron has to be added, then it will experience a repulsive force or columbic force of repulsion. Hence more energy has to be supplied for the addition of an electron to this uninegative ion. Therefore , the second electron gain enthalpy is positive for oxygen.
Answered by Prachi Sawant | 30 Jul, 2014, 02:41: PM
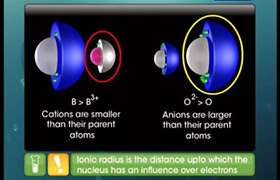
Concept Videos
CBSE 11-science - Chemistry
Asked by dreamaqua2017 | 04 Feb, 2024, 04:25: PM
CBSE 11-science - Chemistry
Asked by kamyabansal2905 | 16 Jul, 2022, 07:11: PM
CBSE 11-science - Chemistry
Asked by jigneshd5127 | 18 Jan, 2021, 09:16: AM
CBSE 11-science - Chemistry
Asked by sulaikhasulu393 | 30 May, 2020, 11:30: AM
CBSE 11-science - Chemistry
Asked by ayushvasava2003 | 09 Mar, 2020, 02:28: PM
CBSE 11-science - Chemistry
Asked by Trisha Gupta | 20 Feb, 2020, 08:47: PM
CBSE 11-science - Chemistry
Asked by Trisha Gupta | 19 Feb, 2020, 08:54: PM
CBSE 11-science - Chemistry
Asked by amulyagampa2002 | 12 Dec, 2018, 11:29: AM
CBSE 11-science - Chemistry
Asked by Ujwalsidhu19 | 08 Nov, 2018, 12:43: PM