ICSE Class 9 Answered
What volume will a gas occupy at 740 mm pressure which at 1480 mm occupies 500 cc ? [ Temperature being constant ]
Ans [ 1000 cc]
Asked by aadrica.kanika | 27 Dec, 2018, 12:12: PM
Given:
P1 =1480 mm
V1 = 500 cc
P2 =740 mm
V2 =?
By Boyle's law
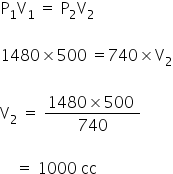
The volume gas is 1000 cc.
Answered by Varsha | 28 Dec, 2018, 10:33: AM
Concept Videos
ICSE 9 - Chemistry
Asked by zainaali39692 | 04 Dec, 2020, 08:53: AM
ICSE 9 - Chemistry
Asked by gup.navya2006 | 01 Dec, 2020, 09:28: AM
ICSE 9 - Chemistry
Asked by Vishusingh2020.2021 | 25 Sep, 2020, 10:09: PM
ICSE 9 - Chemistry
Asked by sudesghnapattanayak2017 | 19 May, 2020, 08:13: PM
ICSE 9 - Chemistry
Asked by abeshchakraborty6 | 23 Feb, 2020, 08:54: AM
ICSE 9 - Chemistry
Asked by dnlwalkers | 08 Jan, 2020, 09:57: AM
ICSE 9 - Chemistry
Asked by raichuratanvi | 14 Dec, 2019, 12:02: PM
ICSE 9 - Chemistry
Asked by merajanjum87 | 21 Nov, 2019, 09:53: PM
ICSE 9 - Chemistry
Asked by parvathimanjunath24 | 31 Oct, 2019, 09:34: PM
ICSE 9 - Chemistry
Asked by devbhaisha.tl | 21 Sep, 2019, 10:02: PM



