CBSE Class 12-science Answered
What is the role of dry ether with Na in reactions.
Asked by rakeshraghav33 | 21 Jan, 2019, 02:49: PM
Sodium metal is highly reactive. Wurtz reaction is carried out with sodium metal.
So solvent is used which will not react with sodium metal.
If the solvent is not used, sodium will react with water to form oxides and hydroxides, which will disturb the reaction.
Dry ether is an aprotic solvent and it also acts as a catalyst.
Answered by Varsha | 21 Jan, 2019, 06:20: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by roshanisharma200611 | 07 Feb, 2024, 01:18: PM
CBSE 12-science - Chemistry
Asked by surekhas66675 | 02 Sep, 2021, 05:17: PM
CBSE 12-science - Chemistry
Asked by nazimb0313 | 02 Sep, 2020, 09:34: AM
CBSE 12-science - Chemistry
Asked by mastertask199 | 13 May, 2020, 04:08: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 03 Feb, 2020, 10:42: PM
CBSE 12-science - Chemistry
Asked by ajaysankhala051 | 11 Sep, 2019, 01:19: PM
CBSE 12-science - Chemistry
Asked by pragyachandraul03 | 23 Aug, 2019, 02:12: PM
CBSE 12-science - Chemistry
Asked by priadkonkar | 21 Jan, 2019, 08:52: PM
CBSE 12-science - Chemistry
Asked by rakeshraghav33 | 21 Jan, 2019, 02:49: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 16 May, 2018, 02:34: PM




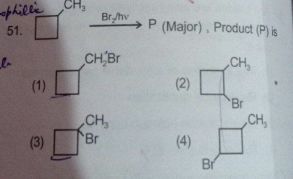
 Total No. of Mono Brominated product :-
Total No. of Mono Brominated product :-