JEE Class main Answered
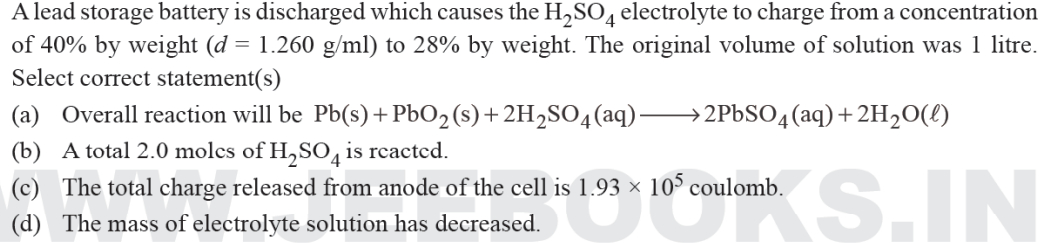
What is the potential of half cell consisting of electrode in solution at 250C(E0oxi=0.763V)
A.
0.8221V
B.
8.221V
C.
0.5282V
D.
9.282V
Asked by s.ojaswini17 | 15 Feb, 2019, 21:09: PM
Option A is correct.
Question:
What is the single electrode potential of half-cell for zinc electrode dipping in 0.01 M ZnSO4 solution at 25 0C?
The standard electrode potential of Zn/Zn2+ is 0.0763 volt.
Given:
n = 2
E0oxid =0.763 V
We know that,
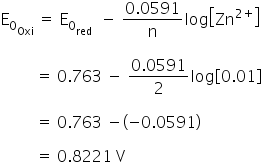
Electrode potential is 0.8221 V
Answered by Varsha | 18 Feb, 2019, 12:53: PM
JEE main - Chemistry
Asked by Balbir | 28 Aug, 2019, 21:05: PM
JEE main - Chemistry
Asked by s.ojaswini17 | 16 Feb, 2019, 13:02: PM
JEE main - Chemistry
Asked by s.ojaswini17 | 15 Feb, 2019, 21:09: PM