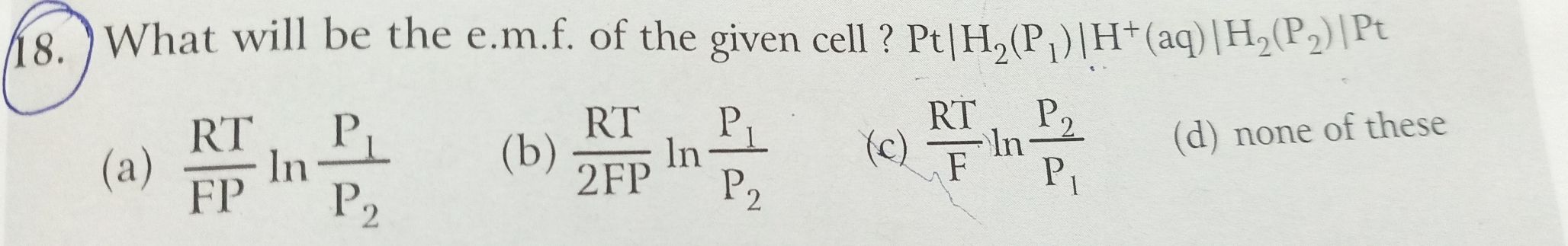JEE Class main Answered
.
Asked by swayamagarwal2114 | 12 Aug, 2022, 10:22: AM
Dear Student,
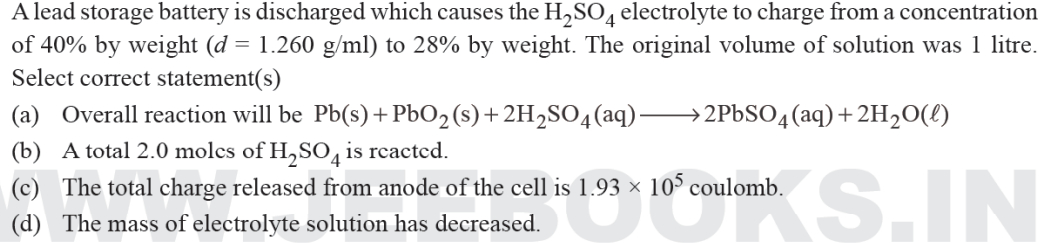
Net cell reaction for the discharge of lead storage battery is:
Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O(l)
y mole y mole
Initial mass of H2SO4,
w1 = 1000 × 1.26 × 40/100 = 504 gm
Final mass of H2SO4,
w2 = (1260 – 98y + 18y) × 28/100 = (352.8 – 22.4y) g
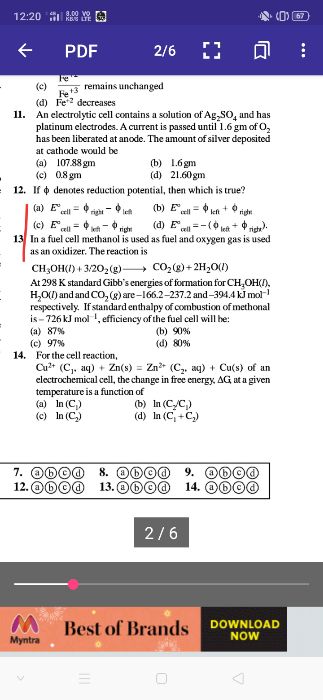
From reaction, 504 – 98y
= 352.8 – 22.4y
y = 2 moles
Thus, final mass w2 = 308 is decreased from initial mass.
No. of moles of H2SO4 reacted,
y = 2 moles
As, 2 moles of electrons have been released in the anode reaction.
Therefore, charge released is 2F
i.e. 2 × 96500 = 1.93 × 105 Coulomb
So, all the given options are correct.
Answered by | 13 Aug, 2022, 10:31: AM
JEE main - Chemistry
Asked by Balbir | 28 Aug, 2019, 21:05: PM
JEE main - Chemistry
Asked by s.ojaswini17 | 16 Feb, 2019, 13:02: PM
JEE main - Chemistry
Asked by s.ojaswini17 | 15 Feb, 2019, 21:09: PM