CBSE Class 12-science Answered
We have not understood the query that you have posted. We would request you to clarify / provide additional details so that we may answer this to the best of the ability.
Benzene reacts at room temperature with a chloroalkane for example, methyl chloride or ethyl chloride etc in the presence of aluminium chloride as a catalyst.
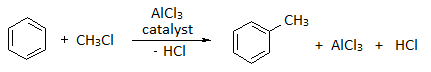

Aluminium chloride, AlCl3, is an electron deficient molecule and is covalently bonded. The aluminium forms three bonds, and has no lone pair of electrons.There are only six electrons around the aluminium atom instead of eight. It takes a chloride ion from the chloromethane, and forms a co-ordinate (dative covalent) bond.
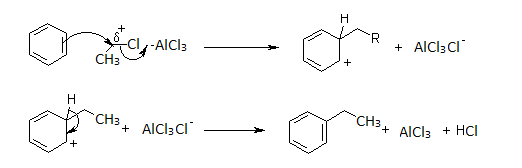
As the CH3+ ion approaches the delocalised electrons in the benzene, electrons get strongly attracted towards the positive charge. Two electrons from the delocalised system are used to form a new bond with the CH3+ ion.
Step 2
The second stage involves the AlCl4-, which is produced at the same time as the CH3+ ion. One of the aluminium-chlorine bonds breaks and both electrons from it are used to join to the hydrogen. This removes the hydrogen from the ring to form HCl, and re-generates the aluminium chloride catalyst in the process. The electrons which originally joined the hydrogen to the ring are now used to re-establish the delocalised system.
Topperlearning Team.









