CBSE Class 12-science Answered
What is a coupling reaction? Give an example.
Asked by Topperlearning User | 04 Jul, 2014, 13:32: PM
Reactions involving retention of Diazo group are coupling reaction. Arenediazonium salts reacts with highly reactive aromatic compounds such as phenols and amines to form brightly coloured azo compounds, Ar-N=N-Ar. The azo products have extended conjugate system having both the aromatic rings joined by N=N bonds. Mostly such compounds are used in the manufacture of dyes, as this compounds are coloured in nature. For example benzene diazonium chloride reacts with phenol to give an orange dye i.e. p-Hydroxyazobenzene. 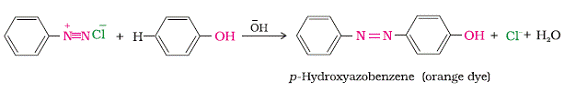
Answered by | 04 Jul, 2014, 15:32: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by rajagopalmanoj08 | 20 Dec, 2019, 21:59: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 06 Jun, 2016, 11:05: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jul, 2014, 13:32: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jul, 2014, 13:45: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 06 Jun, 2016, 11:06: AM





