CBSE Class 12-science Answered
what do you mean polar and non polar substances how they affect by the presence of electric field
Asked by solankiarpit55 | 09 Feb, 2021, 06:32: AM
Polar substances are made up of polar molecules . Non-polar substances are made up of non-polar molecules .
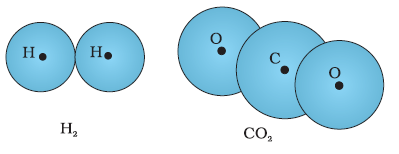
In a non-polar molecule, the centres of positive and negative charges coincide. The molecule then has no
permanent (or intrinsic) dipole moment. Examples of non-polar molecules are oxygen (O2) and hydrogen (H2)
molecules which, because of their symmetry, have no dipole moment.
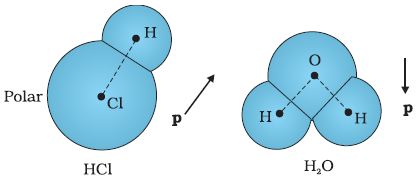
On the other hand, a polar molecule is one in which the centres of positive and negative charges are
separated (even when there is no external field). Such molecules have a permanent dipole moment.
An ionic molecule such as HCl or a molecule of water (H2O) are examples of polar molecules.
In an external electric field, the positive and negative charges of a nonpolar molecule are displaced in opposite
directions. The displacement stops when the external force on the constituent charges of the molecule is balanced by
the restoring force (due to internal fields in the molecule). The non-polar molecule thus develops an induced dipole moment.
The dielectric is said to be polarised by the external field. The induced dipole moments of different molecules add up giving a net
dipole moment of the dielectric in the presence of the external field.
directions. The displacement stops when the external force on the constituent charges of the molecule is balanced by
the restoring force (due to internal fields in the molecule). The non-polar molecule thus develops an induced dipole moment.
The dielectric is said to be polarised by the external field. The induced dipole moments of different molecules add up giving a net
dipole moment of the dielectric in the presence of the external field.
A dielectric with polar molecules also develops a net dipole moment in an external field, but this effect
of polar molecule is different from non-polar molecules. In the absence of any external field, the different permanent
dipoles are oriented randomly due to thermal agitation; so the total dipole moment is zero. When
an external field is applied, the individual dipole moments tend to align with the field. When summed over all the molecules,
an external field is applied, the individual dipole moments tend to align with the field. When summed over all the molecules,
there is then a net dipole moment in the direction of the external field, i.e., the dielectric is polarised.
The extent of polarisation depends on the relative strength of two mutually opposite factors: the dipole potential energy
in the external field tending to align the dipoles with the field and thermal energy tending to disrupt the alignment.
There may be displacement of positive and negative charges due to external electric field in polar molecules,
but generally the alignment effect is more important for polar molecules.
Thus in either case, whether polar or non-polar, a dielectric develops a net dipole moment in the presence of an external field.
Thus in either case, whether polar or non-polar, a dielectric develops a net dipole moment in the presence of an external field.
Answered by Thiyagarajan K | 09 Feb, 2021, 09:08: PM
Concept Videos
CBSE 12-science - Physics
Asked by ankush76728 | 06 May, 2024, 04:52: PM
CBSE 12-science - Physics
Asked by ankush76728 | 05 May, 2024, 09:55: PM
CBSE 12-science - Physics
Asked by heymindurownbusiness | 04 May, 2024, 11:15: AM
CBSE 12-science - Physics
Asked by talulu | 01 May, 2024, 05:14: PM
CBSE 12-science - Physics
Asked by kanishkg511 | 30 Apr, 2024, 07:25: PM
CBSE 12-science - Physics
Asked by sahoobanita89 | 30 Apr, 2024, 05:10: AM
CBSE 12-science - Physics
Asked by divakar.9124 | 27 Apr, 2024, 10:42: PM
CBSE 12-science - Physics
Asked by panneer1766 | 24 Apr, 2024, 01:52: PM