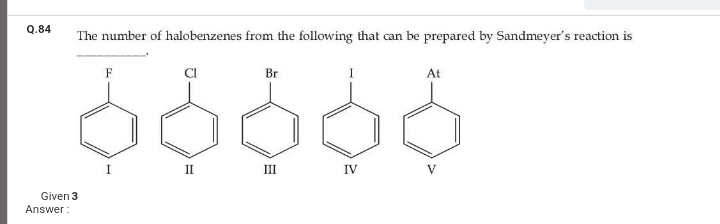
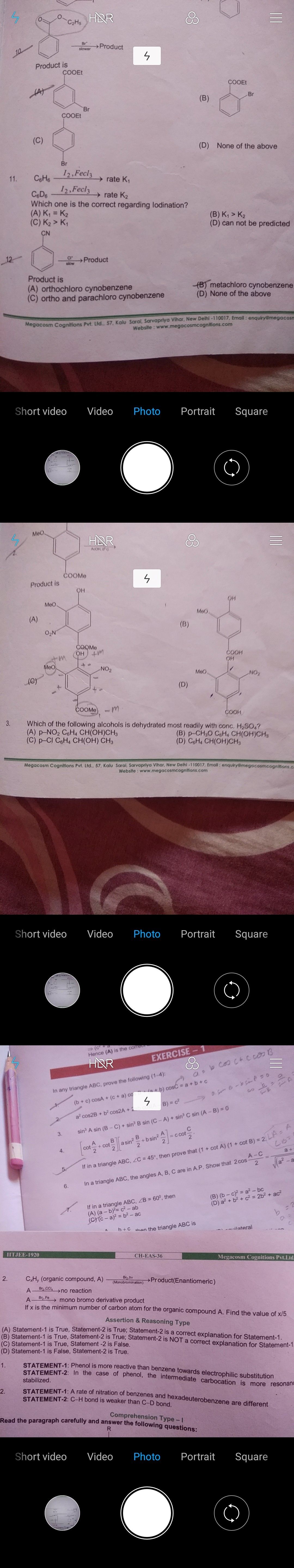
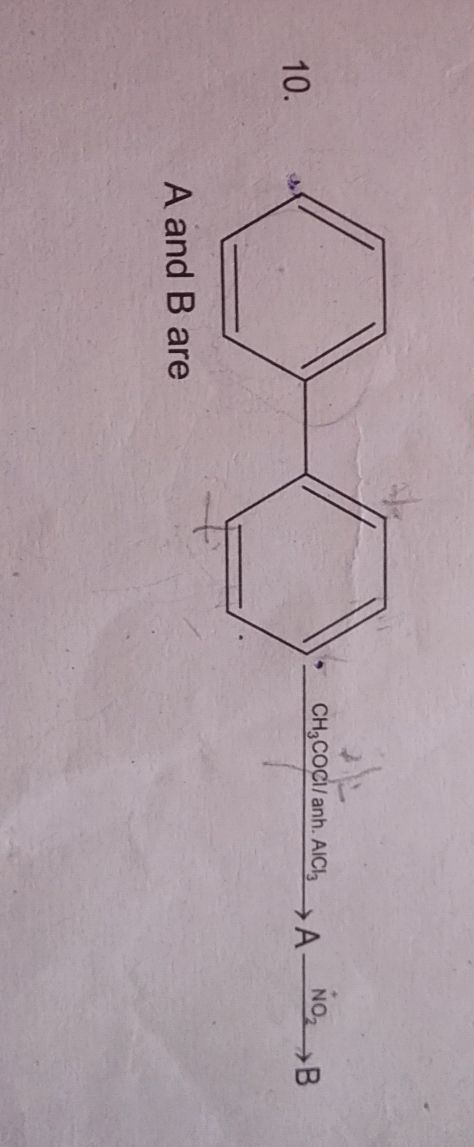
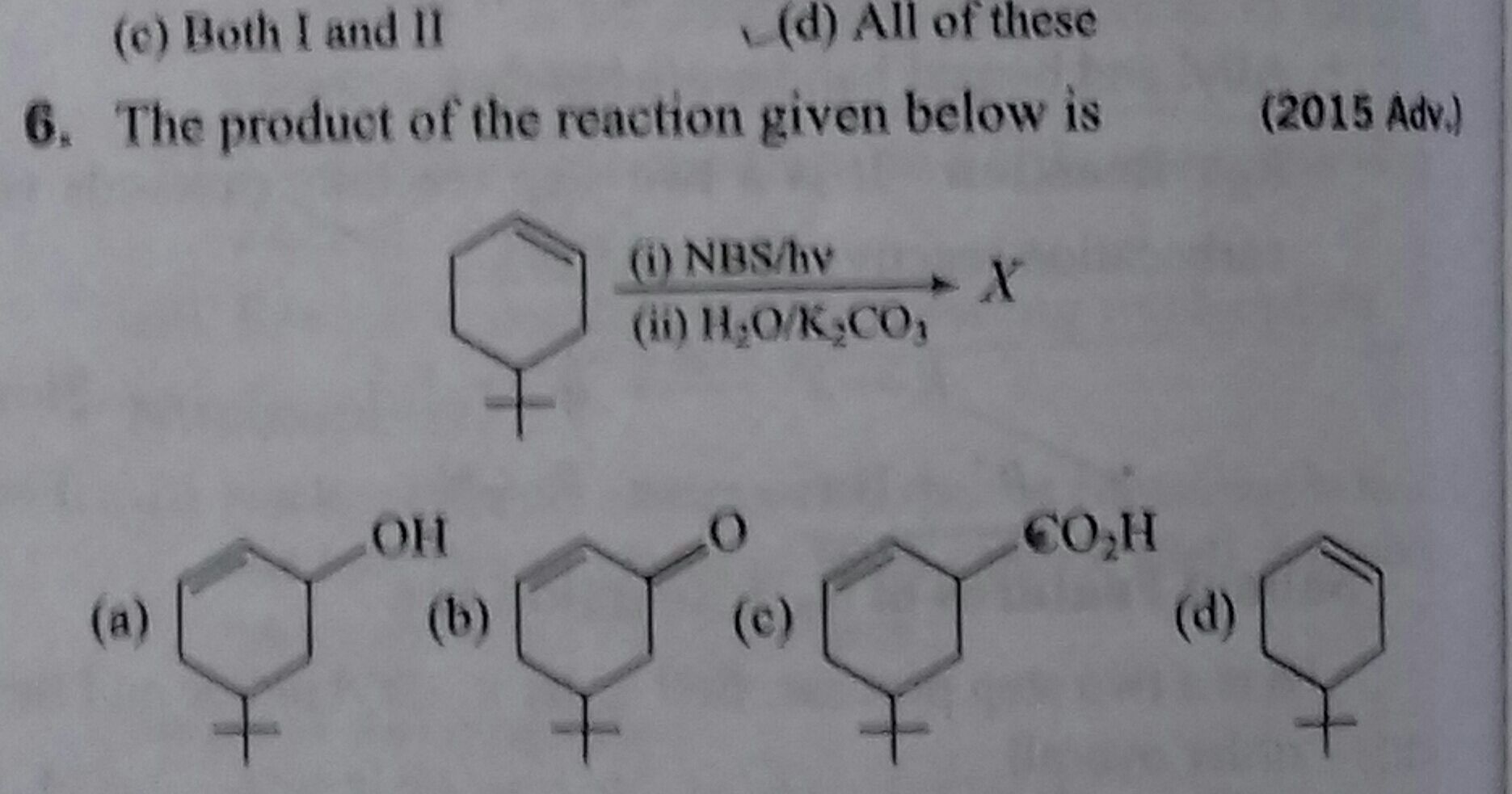JEE Class main Answered
The stability of interhalogen compounds follows the
order
(a) CIF₃, > BrF₃, >IF₃ (b) BrF₃ > IF₃, > CIF₃:
(C) IF₃, > BrF₃, > CIF₃ (d) CIF₃, > IF₃, > BrF₃;

Asked by hemeshsaini2005 | 11 Oct, 2021, 06:13: PM
Answer C
Solution: Electropositive character of halogen is in order I >Br>Cl. Central atom is bigger in size, more electropositive nature form stable interhalogen compound.
Answered by Ramandeep | 12 Oct, 2021, 02:48: PM
JEE main - Chemistry
Asked by muppanenicharitha | 14 Apr, 2024, 08:23: PM
JEE main - Chemistry
Asked by gourishettikrishna | 25 Jan, 2024, 10:01: PM
JEE main - Chemistry
Asked by hemeshsaini2005 | 11 Oct, 2021, 06:13: PM
JEE main - Chemistry
Asked by yasharthshankar | 30 Jun, 2020, 11:13: PM
JEE main - Chemistry
Asked by mdfaraz1182 | 09 Apr, 2020, 11:10: AM
JEE main - Chemistry
Asked by jhajuhi19 | 29 Mar, 2020, 11:19: AM
JEE main - Chemistry
Asked by vidyavikram10 | 29 Mar, 2020, 11:09: AM
JEE main - Chemistry
Asked by rsudipto | 24 Dec, 2018, 09:02: AM