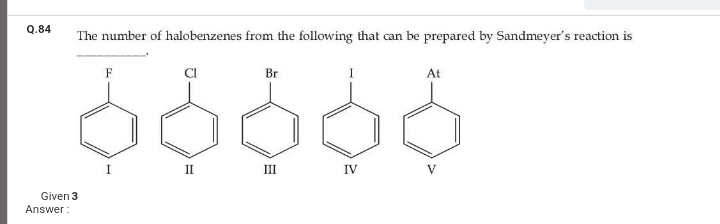
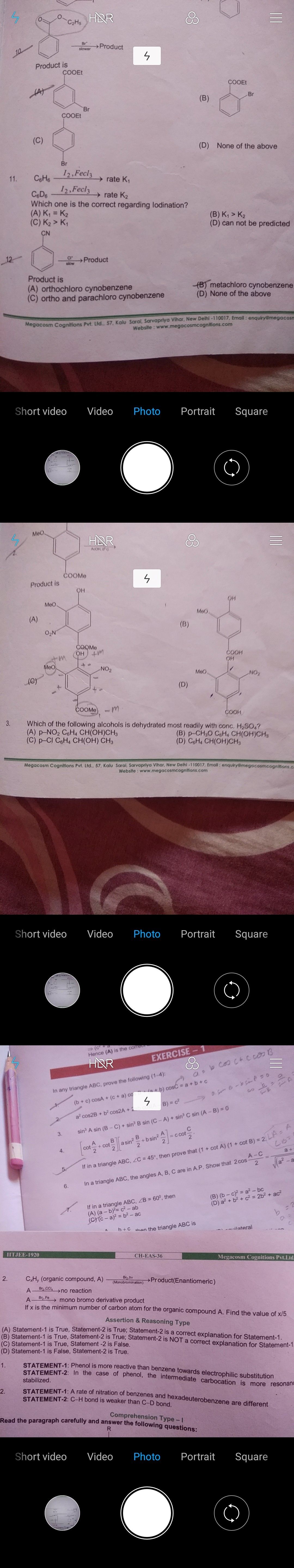
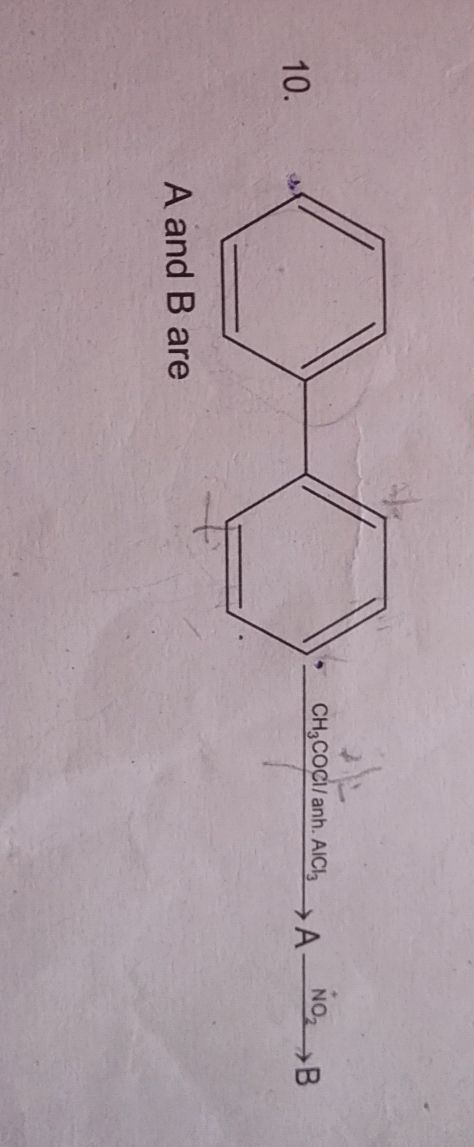
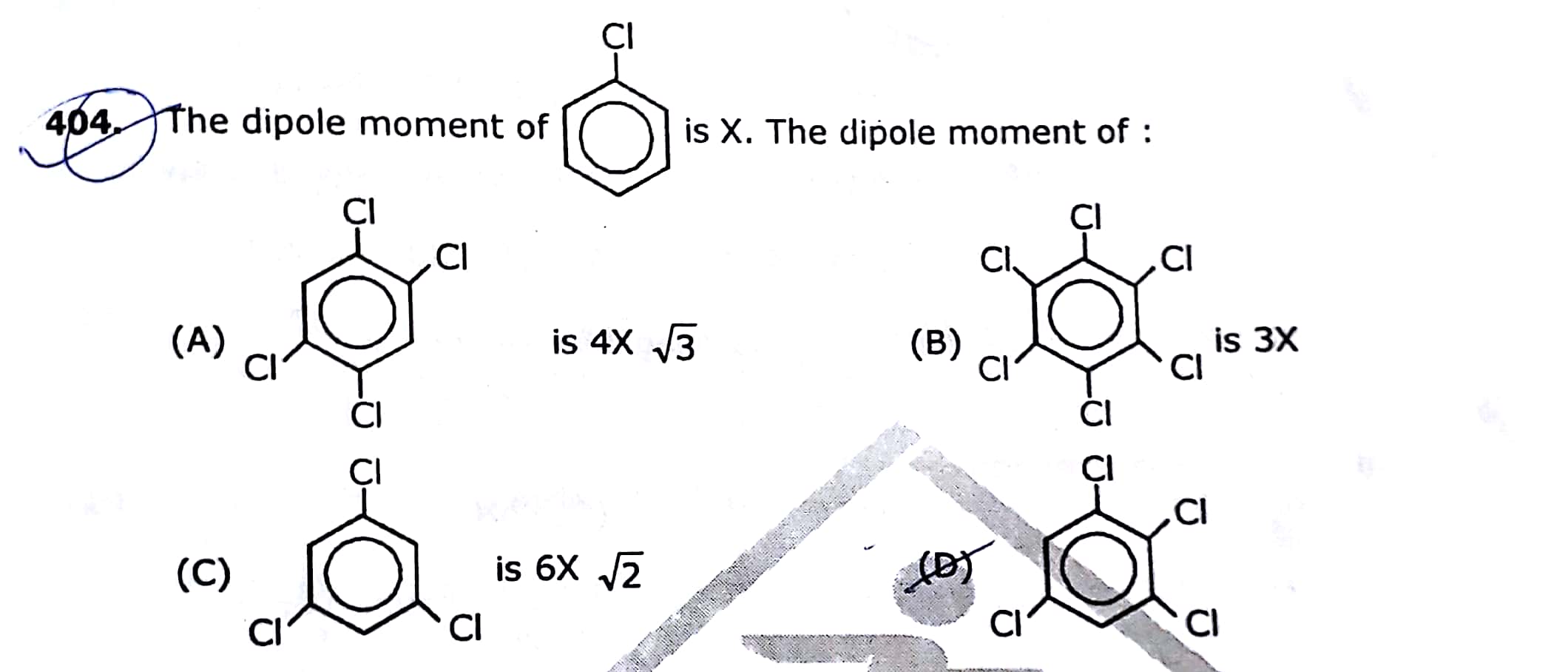
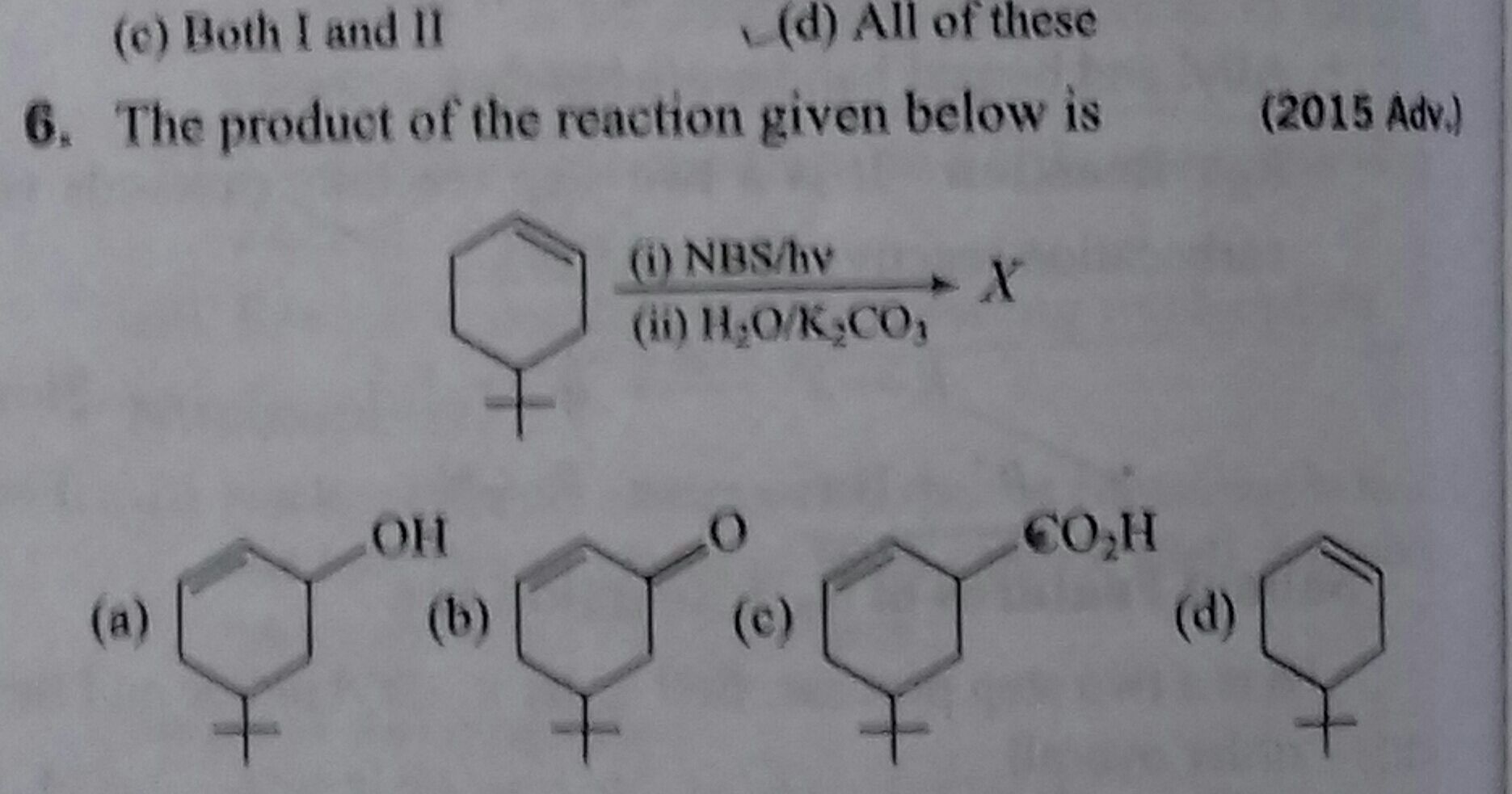JEE Class main Answered
Dear Student,
Chlorination of chlorobenzene with chlorine in the presence of anhydrous 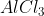 gives a mixture of ortho and para isomers of dichlorobenzene. Because of the differences in carbon and chlorine's electronegativities, chlorine exhibits the -I effect in aliphatic chains. chlorine has stronger electronegativity, or the capacity to draw in the shared pair of electrons. In the benzene ring, specifically in chlorobenzene, chlorine displays both the resonance effect (+R) and -I. While the delocalization of a lone pair provides electron density towards the ring, the inductive effect removes electrons. The positive charge that develops on the ortho or para position during the electrophilic substitution reaction is destabilized due to the inductive effect.However, the electron density at those sites is also increased by the lone pair on chlorine. Delocalization of the lone pair in the ring stabilizes the carbocation produced during the reaction. Since the +R effect in this instance is less than the -I effect.
gives a mixture of ortho and para isomers of dichlorobenzene. Because of the differences in carbon and chlorine's electronegativities, chlorine exhibits the -I effect in aliphatic chains. chlorine has stronger electronegativity, or the capacity to draw in the shared pair of electrons. In the benzene ring, specifically in chlorobenzene, chlorine displays both the resonance effect (+R) and -I. While the delocalization of a lone pair provides electron density towards the ring, the inductive effect removes electrons. The positive charge that develops on the ortho or para position during the electrophilic substitution reaction is destabilized due to the inductive effect.However, the electron density at those sites is also increased by the lone pair on chlorine. Delocalization of the lone pair in the ring stabilizes the carbocation produced during the reaction. Since the +R effect in this instance is less than the -I effect.