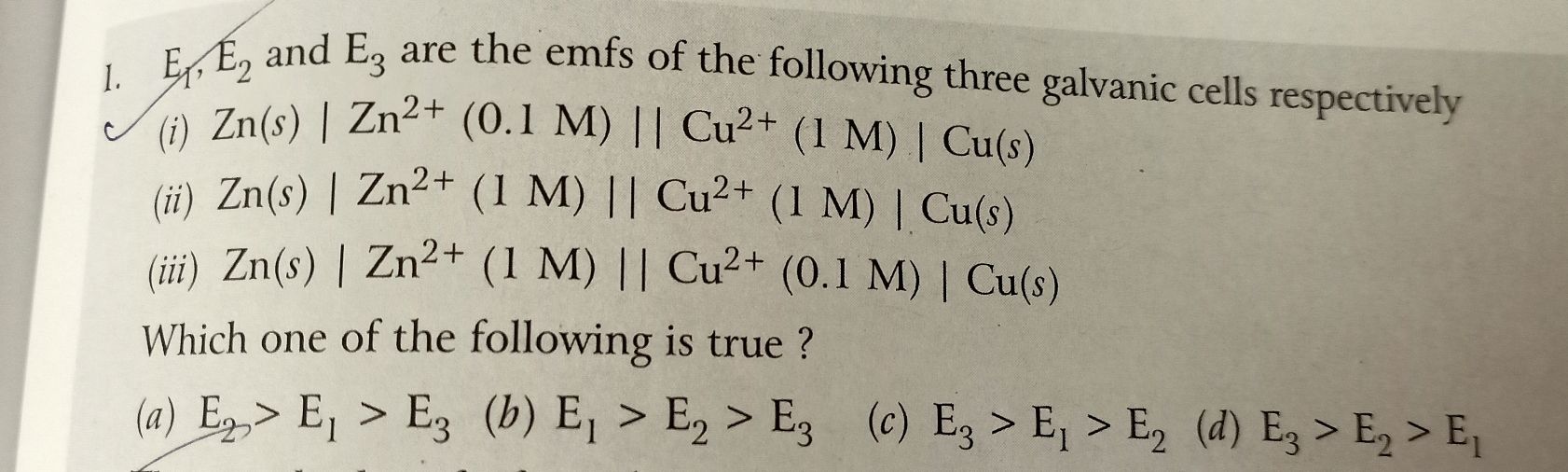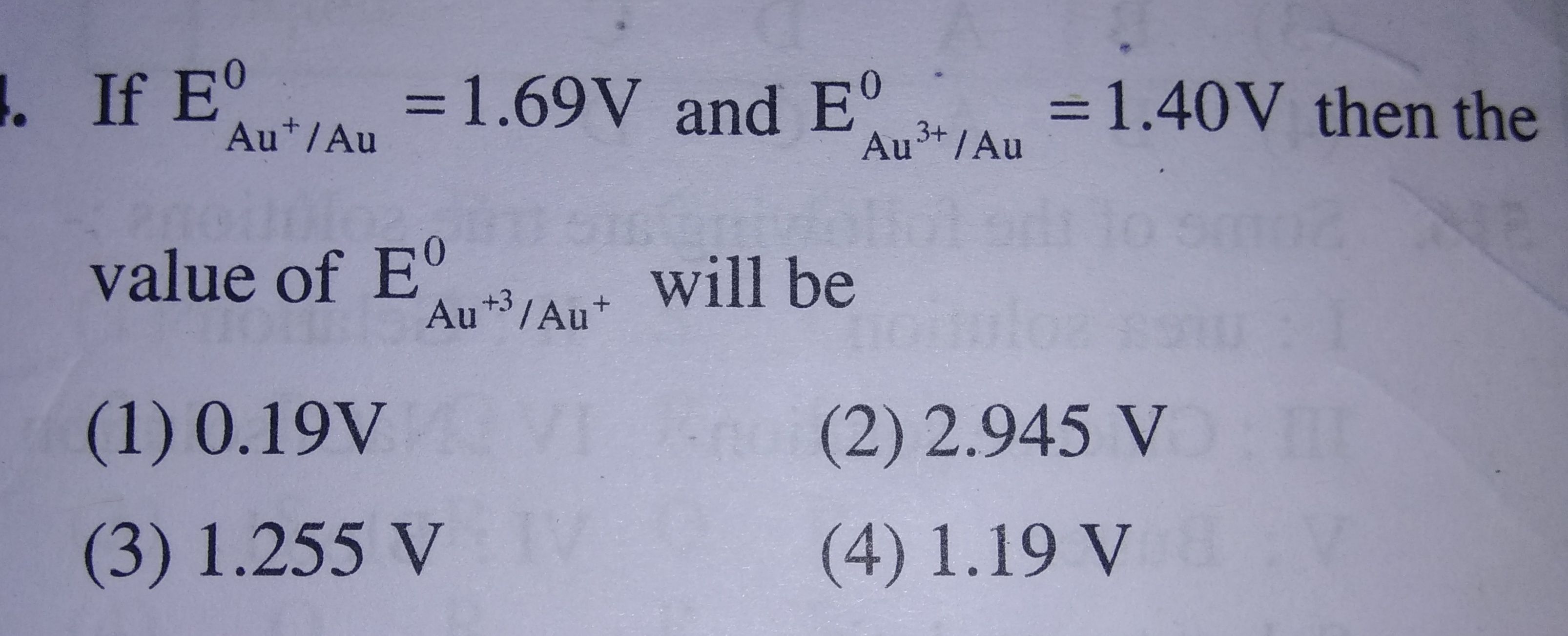CBSE Class 12-science Answered
The discharge potential of Chloride ions is less than hydroxide ions . what does this line mean and why chloride has less discharge potential.
Asked by govtsecschoolnayaganv051 | 05 Nov, 2019, 04:50: PM
If an electrolytic solution consists of more than two ions and the electrolysis is done then these ions are reduced or oxidised on electrodes. The ions yu asked are anions. These ions will oxidised at anode. So in this electric discharge, discharge potential is oxidation potential. If it was reduction then in that case it would be reduction potential. OH- has relatively high oxidation potential So discharge potential of chloride is less than hydroxide ions.
Answered by Ravi | 08 Nov, 2019, 06:34: PM
Concept Videos
CBSE 12-science - Chemistry
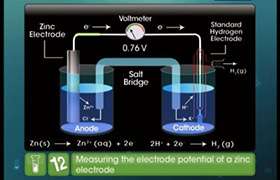
Asked by rajpatil | 19 Jun, 2020, 07:12: PM
CBSE 12-science - Chemistry
Asked by piyushkhariyal | 06 Mar, 2020, 10:36: PM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 15 Feb, 2020, 08:21: PM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 11 Nov, 2019, 12:09: AM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 05 Nov, 2019, 04:50: PM
CBSE 12-science - Chemistry
Asked by Balbir | 21 Aug, 2019, 09:14: PM
CBSE 12-science - Chemistry
Asked by ankitathapliyal097 | 24 Jul, 2019, 10:05: PM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 26 Jun, 2019, 02:18: PM
CBSE 12-science - Chemistry
Asked by jhajuhi19 | 01 May, 2019, 09:03: PM
CBSE 12-science - Chemistry
Asked by pardeepkumar2281 | 26 Sep, 2018, 09:00: PM