JEE Class main Answered
The answer given here is (d). But shouldn't the actual answer be (c), because hyperconjugation doesn't operate at meta, hence (c) has more electron density?
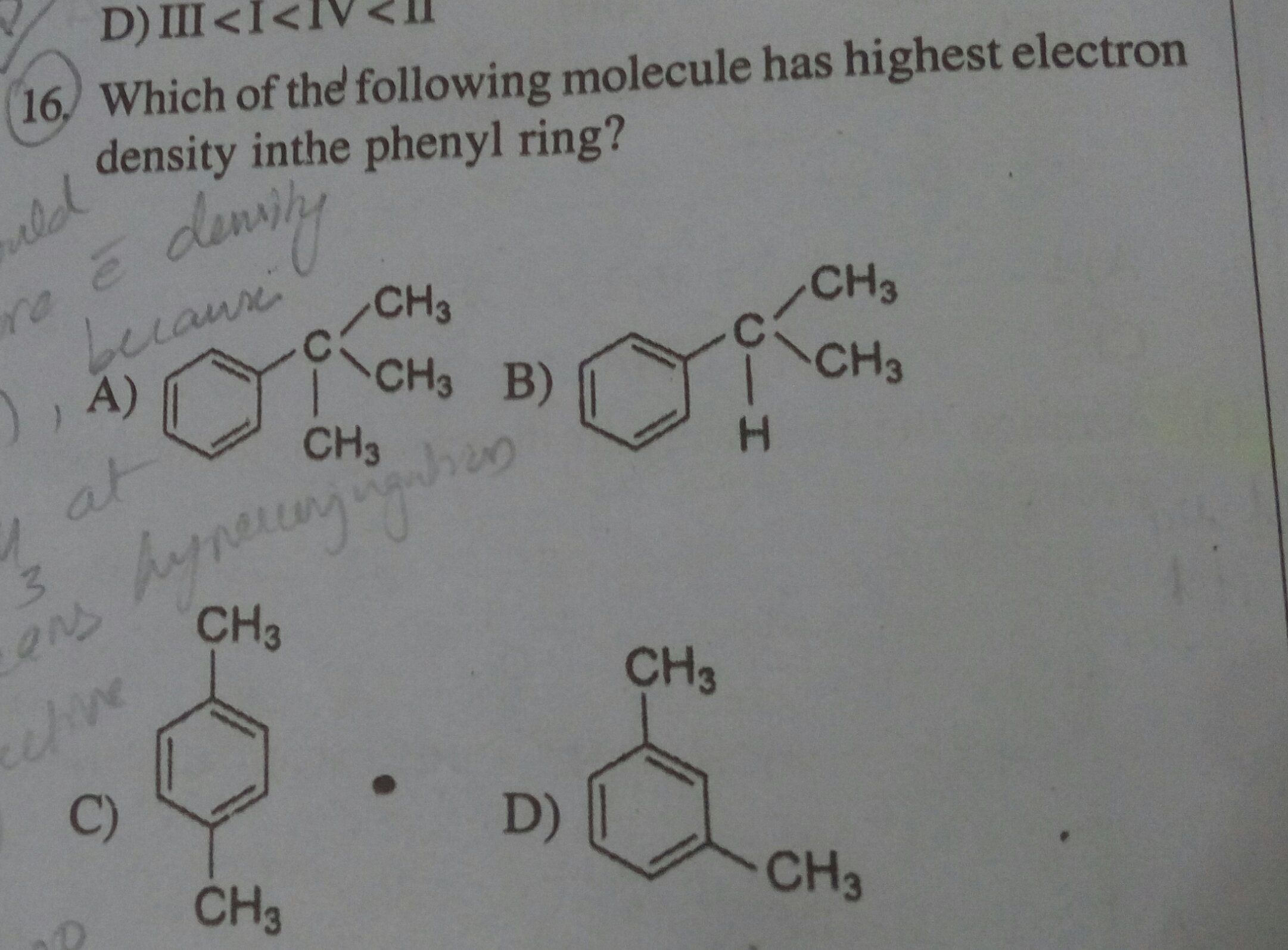
Asked by sumayiah2000 | 04 May, 2019, 12:16: PM
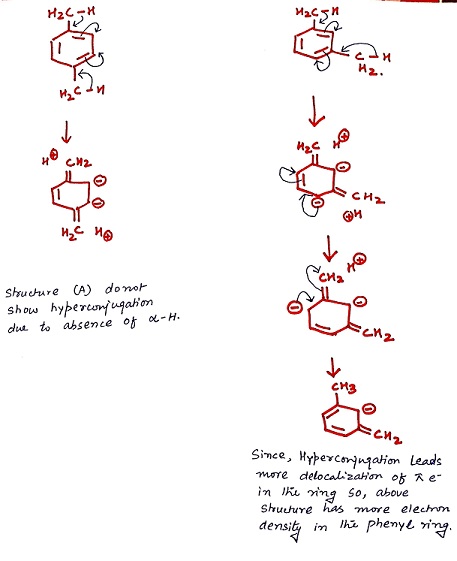
Option c is not the answer because in the structure shown above two -ve charges are at the adjecent carbon atoms which makes the molecule unstable.
Magnitude of delocalization of electrons across the phenyl ring decides the electron density thus answer given is option "d".
Answered by Sumit Chakrapani | 06 May, 2019, 12:45: PM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by neerajavuppala1983 | 23 Jul, 2024, 22:49: PM
JEE main - Chemistry
Asked by tanniruv133 | 03 Jul, 2024, 18:50: PM
JEE main - Chemistry
Asked by heyyyyy | 12 Jun, 2024, 19:18: PM
JEE main - Chemistry
Asked by hv5594265 | 12 Jun, 2024, 11:59: AM
JEE main - Chemistry
Asked by rupalibhange1987 | 11 Jun, 2024, 20:00: PM
JEE main - Chemistry
Asked by gajju8493 | 11 Jun, 2024, 15:09: PM
JEE main - Chemistry
Asked by chakrabortymithu041 | 29 May, 2024, 18:45: PM













