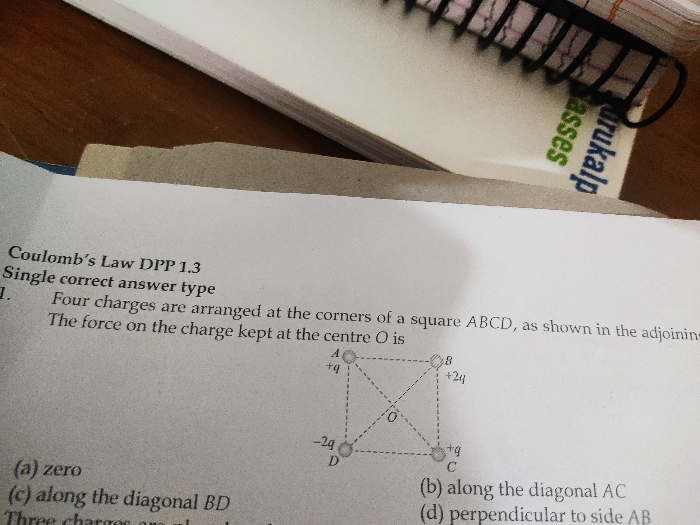JEE Class main Answered
Tell Me
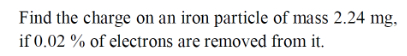
Asked by choudharylucky155 | 22 Apr, 2023, 17:06: PM
Atomic weight of iron = 55.845 amu ;
Number n of atoms in 2.24 mg iron is
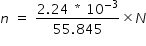
Where N = 6.02 × 1023 is avagadro number .
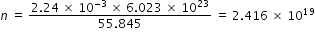
Number of electrons removed , ne = 2.416 × 1019 × 0.02 × 10-2 = 4.832 × 1015
Charge removed from given quantity of iron = -4.832 × 1015 × 1.602 × 10-19 = -7.741 × 10-4 C
Charge on given 2.24 mg iron = + 7.741 × 10-4 C
---------------------------------------------------------------------------------------------------
Answered by Thiyagarajan K | 22 Apr, 2023, 20:41: PM
JEE main - Physics
Asked by manishkanna555 | 07 Jul, 2024, 10:25: AM
JEE main - Physics
Asked by chandana9827 | 13 Jun, 2024, 20:28: PM
JEE main - Physics
Asked by ratnadeep.dmr003 | 21 Apr, 2024, 23:06: PM
JEE main - Physics
Asked by medhamahesh007 | 02 Apr, 2024, 11:11: AM
JEE main - Physics
Asked by chhayasharma9494 | 31 Mar, 2024, 12:47: PM
JEE main - Physics
Asked by archithateja3 | 30 Mar, 2024, 22:23: PM
JEE main - Physics
Asked by mfkatagi099 | 20 Mar, 2024, 21:35: PM