JEE Class main Answered
solve all the three paragraph questions in detail
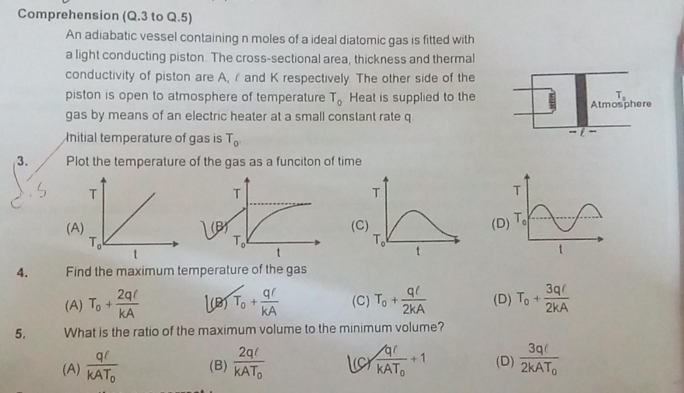
Asked by sarveshvibrantacademy | 11 May, 2019, 11:58: AM
At steady state we have the heat conduction equation across the piston , q = k A (dT/l) = k A (T-T0)/l .......................(1)
where q is steady state heat flux, k is thermal conductivity of material of piston, A is area of piston,
T0 is initial temperature and T is final temperature.
we rewrite eqn.(1) as T = T0 + ql/(kA) .................(2)
Qn.(3)
initially heat flux q = 0 and steadily it is increasing to reach constant value.
Hence Temperature of enclosed gas steadily increasing staring from T0 and reaching steady state value T as shown in fig.(B)
Qn(4) maximum temperature is as given in eqn.(2)
Qn.(5) since otherside of piston at atmospheric pressure, pressure of the enclosed gas is constant.
For ideal gas at constant pressure, we have V/T = constant, where V is volume.
hence V/V0 = T/T0 .........................(3)
by dividing both side of eqn.(2) by T0 we get ( T/T0 ) = 1 + [ ql/(k A T0) ] = V/V0
Answered by Thiyagarajan K | 11 May, 2019, 16:26: PM
Application Videos
Concept Videos
JEE main - Physics
Asked by sumalathamadarapu9 | 23 Oct, 2024, 22:06: PM
JEE main - Physics
Asked by py309649 | 13 Oct, 2024, 13:39: PM
JEE main - Physics
Asked by coolskrish | 13 Oct, 2024, 12:50: PM
JEE main - Physics
Asked by midnightmoon3355 | 09 Oct, 2024, 09:09: AM
JEE main - Physics
Asked by rambabunaidu4455 | 03 Oct, 2024, 16:03: PM
JEE main - Physics
Asked by ratchanavalli07 | 17 Sep, 2024, 07:46: AM
JEE main - Physics
Asked by yayashvadutta45 | 15 Sep, 2024, 19:47: PM
JEE main - Physics
Asked by adithireddy999 | 03 Sep, 2024, 09:35: AM
JEE main - Physics
Asked by vaishalinirmal739 | 29 Aug, 2024, 18:07: PM
JEE main - Physics
Asked by vradhysyam | 26 Aug, 2024, 17:17: PM