JEE Class main Answered
Sir,Why these 3 compounds don't react with LiAlH4,CH3MgBr and KOH ??If u can show some reason with mechanisms type...it will be pleasurable for me,thanks
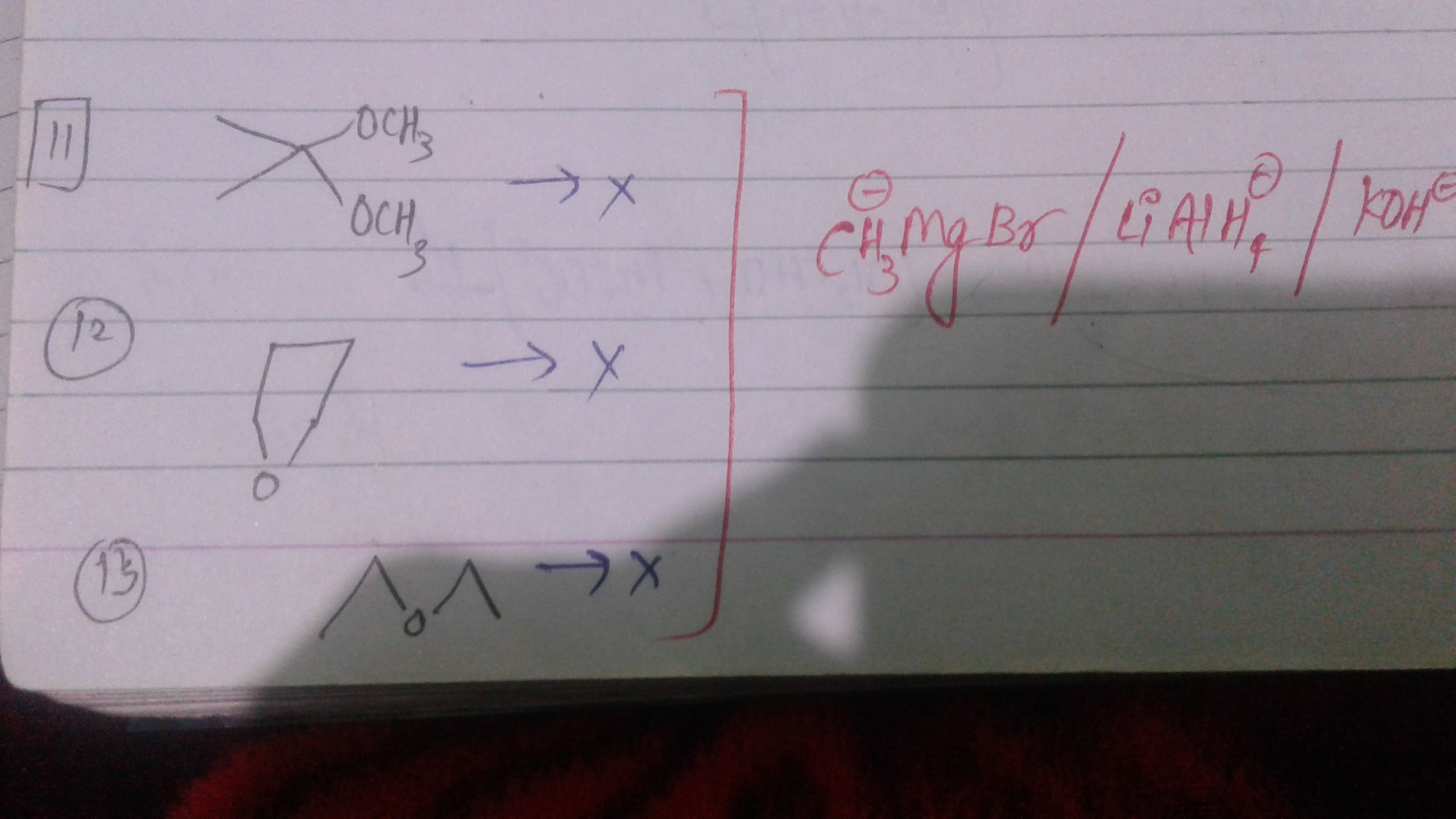
Asked by vishakhachandan026 | 22 Jan, 2020, 10:08: AM
In the first reactions, the given reactant is a ketone whose carbonyl group is protected by alcohol, hence it does not react with the Grignard reagent.

Since the reaction centre carbonyl group is protected by a protecting group i.e. alcohol, the nucleophile or Grignard reagent will not give any reaction.
The same case is with reaction (2) and (3), because of protecting group that specific functional group is protected and does not give reaction further.
following is an example for better understanding:
In the below reaction if we treat strong reducing agent LiAlH4 with the reactant it reduces both ketones as well as ester, well if we only want to reduce ester not ketone then with the help of protecting group glycol this can be possible.
Glycol is used as a protecting group for the carbonyl group, which can easily be removed by simply hydrolysis.

Answered by Ramandeep | 22 Jan, 2020, 11:37: AM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by neerajavuppala1983 | 23 Jul, 2024, 22:49: PM
JEE main - Chemistry
Asked by tanniruv133 | 03 Jul, 2024, 18:50: PM
JEE main - Chemistry
Asked by heyyyyy | 12 Jun, 2024, 19:18: PM
JEE main - Chemistry
Asked by hv5594265 | 12 Jun, 2024, 11:59: AM
JEE main - Chemistry
Asked by rupalibhange1987 | 11 Jun, 2024, 20:00: PM
JEE main - Chemistry
Asked by gajju8493 | 11 Jun, 2024, 15:09: PM
JEE main - Chemistry
Asked by chakrabortymithu041 | 29 May, 2024, 18:45: PM













