JEE Class main Answered
Question no 29
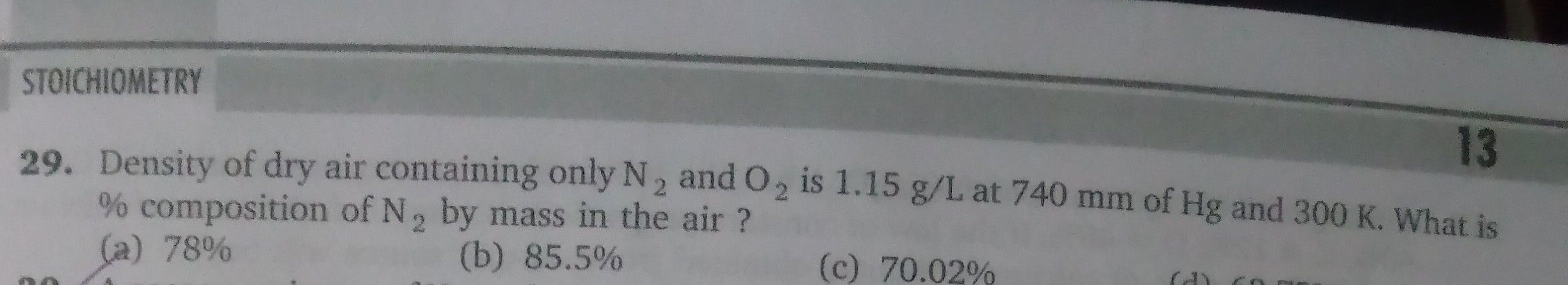
Asked by Adityaaryan681681 | 30 Jul, 2019, 22:15: PM
Given:
Density = 1.15 g/L
P = 740 mm Hg

T = 300 K
Using ideal gas equation,
PV = nRT

Let % of N2 be X and O2 be (1-X)
Therefore,
28X + (1-X)32 = 29.09
28X + 32- 32X = 29.09
-4X = -2.90
X = 0.7274
=72.74 % of N2
Composition of N2 is 72%
Answered by Varsha | 31 Jul, 2019, 11:01: AM
JEE main - Chemistry
Asked by Mdizhanshaikh | 20 May, 2024, 19:18: PM
JEE main - Chemistry
Asked by manishguptaballia.15 | 11 May, 2024, 15:57: PM
JEE main - Chemistry
Asked by ashwinskrishna2006 | 18 Apr, 2024, 17:37: PM
JEE main - Chemistry
Asked by gmafia618 | 04 Apr, 2024, 20:48: PM
JEE main - Chemistry
Asked by jadhavshivtej256 | 27 Feb, 2024, 18:25: PM
JEE main - Chemistry
Asked by pradumankumarsah1 | 30 Jan, 2024, 14:36: PM
JEE main - Chemistry
Asked by srujan11042008 | 06 Nov, 2023, 10:31: AM





