CBSE Class 9 Answered
Question 1.
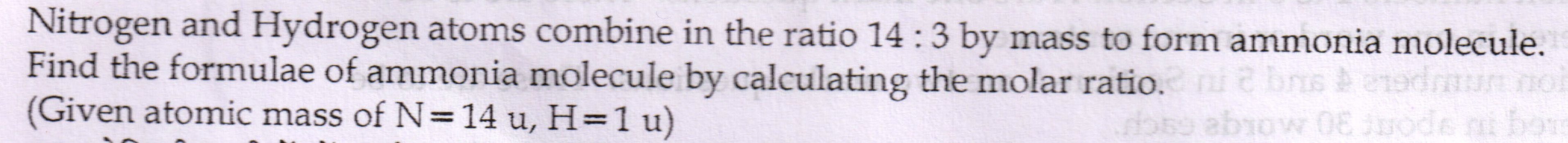
Asked by gpnkumar0 | 13 Mar, 2017, 04:55: PM
(1) Mass ratio (N:H) =14:3
(2) Atomic ratio = Mass ratio/Relative atomic mass
(3) Simple ratio
|
Element |
Mass ratio |
Atomic ratio |
Simple ratio |
|
Nitrogen |
14 |
14/14=1 |
1 |
|
Hydrogen |
3 |
3/1=3 |
3 |
Chemical formula = N1H3 = NH3
Answered by Vaibhav Chavan | 13 Mar, 2017, 09:09: PM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by rajputanaji290 | 03 Oct, 2023, 09:30: PM
CBSE 9 - Chemistry
Asked by muditsharma287 | 09 Mar, 2023, 10:10: PM
CBSE 9 - Chemistry
Asked by shivalaxmi0205 | 08 Mar, 2023, 07:46: PM
CBSE 9 - Chemistry
Asked by shivalaxmi0205 | 08 Mar, 2023, 07:43: PM
CBSE 9 - Chemistry
Asked by jssjj | 19 Jan, 2023, 07:25: PM
CBSE 9 - Chemistry
Asked by mohammedhaqqani.6b | 14 Jun, 2022, 02:51: PM
CBSE 9 - Chemistry
Asked by jiyajthakor | 28 Feb, 2022, 07:03: PM
CBSE 9 - Chemistry
Asked by gillsaabjashanpreetsingh3 | 16 Jan, 2022, 01:23: PM
CBSE 9 - Chemistry
Asked by prachisharma772007 | 16 Jan, 2022, 11:12: AM
CBSE 9 - Chemistry
Asked by rupasajwan363 | 04 Jan, 2022, 04:17: PM








