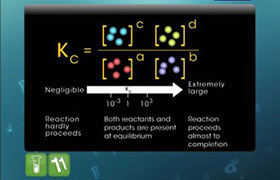
JEE Class main Answered
Q) The degree of dissociation of PCL5 at one atmospheric pressure is 0.2. Calculate the pressure at which PCL5 is dissociated to 50%.
Asked by Anish | 07 Feb, 2019, 17:18: PM
PCl5 dissociates as:
PCl5 ⇌ PCl3 Cl2
If α is the degree of dissociation at any temperature
PCl5 ⇌ PCl3 Cl2
If α is the degree of dissociation at any temperature
under atmospheric pressure, so,
Initially, the concentration will be:
PCl5 = 1
PCl3 = 0
Cl2 = 0
At Equilibrium:
PCl5 = 1 – α
PCl3 = α
Cl2 = α
Total number of moles at equilibrium = 1 – α + α + α = 1 + α
Partial pressures of PCl5, PCl3 and Cl2 will be:
p (PCl3) = α p / 1 + α
p (Cl2) = α p / 1 + α
p (PCl5) = (1 – α) p / 1 + α
Kp = p (PCl3) X p (Cl2) / p (PCl5)
Kp = [(α p / 1 + α) X (α p / 1 + α)]/[ (1 – α) p / (1 + α)]
= α2 p / (1 – α) 2
Partial pressures of PCl5, PCl3 and Cl2 will be:
p (PCl3) = α p / 1 + α
p (Cl2) = α p / 1 + α
p (PCl5) = (1 – α) p / 1 + α
Kp = p (PCl3) X p (Cl2) / p (PCl5)
Kp = [(α p / 1 + α) X (α p / 1 + α)]/[ (1 – α) p / (1 + α)]
= α2 p / (1 – α) 2
Substituting p = 1 atm and α = 0.2
Kp = (0.2) 2 X 1 / (1 – (0.2)) 2
= 0.041
When α = 1/2 = 0.5, let pressure is p’
Kp = α2 p’ / (1 – α) 2
0.041 = (0.5) sup>2 p’ / (1 – (0.5)) 2
p’ = (0.041) [1 – (0.5) 2] / (0.5) 2
p’ = 0.125 atm
Kp = (0.2) 2 X 1 / (1 – (0.2)) 2
= 0.041
When α = 1/2 = 0.5, let pressure is p’
Kp = α2 p’ / (1 – α) 2
0.041 = (0.5) sup>2 p’ / (1 – (0.5)) 2
p’ = (0.041) [1 – (0.5) 2] / (0.5) 2
p’ = 0.125 atm
Answered by Science Mate | 07 Feb, 2019, 19:16: PM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by neerajavuppala1983 | 23 Jul, 2024, 22:49: PM
JEE main - Chemistry
Asked by tanniruv133 | 03 Jul, 2024, 18:50: PM
JEE main - Chemistry
Asked by heyyyyy | 12 Jun, 2024, 19:18: PM
JEE main - Chemistry
Asked by hv5594265 | 12 Jun, 2024, 11:59: AM
JEE main - Chemistry
Asked by rupalibhange1987 | 11 Jun, 2024, 20:00: PM
JEE main - Chemistry
Asked by gajju8493 | 11 Jun, 2024, 15:09: PM
JEE main - Chemistry
Asked by chakrabortymithu041 | 29 May, 2024, 18:45: PM