JEE Class main Answered
Plz answer
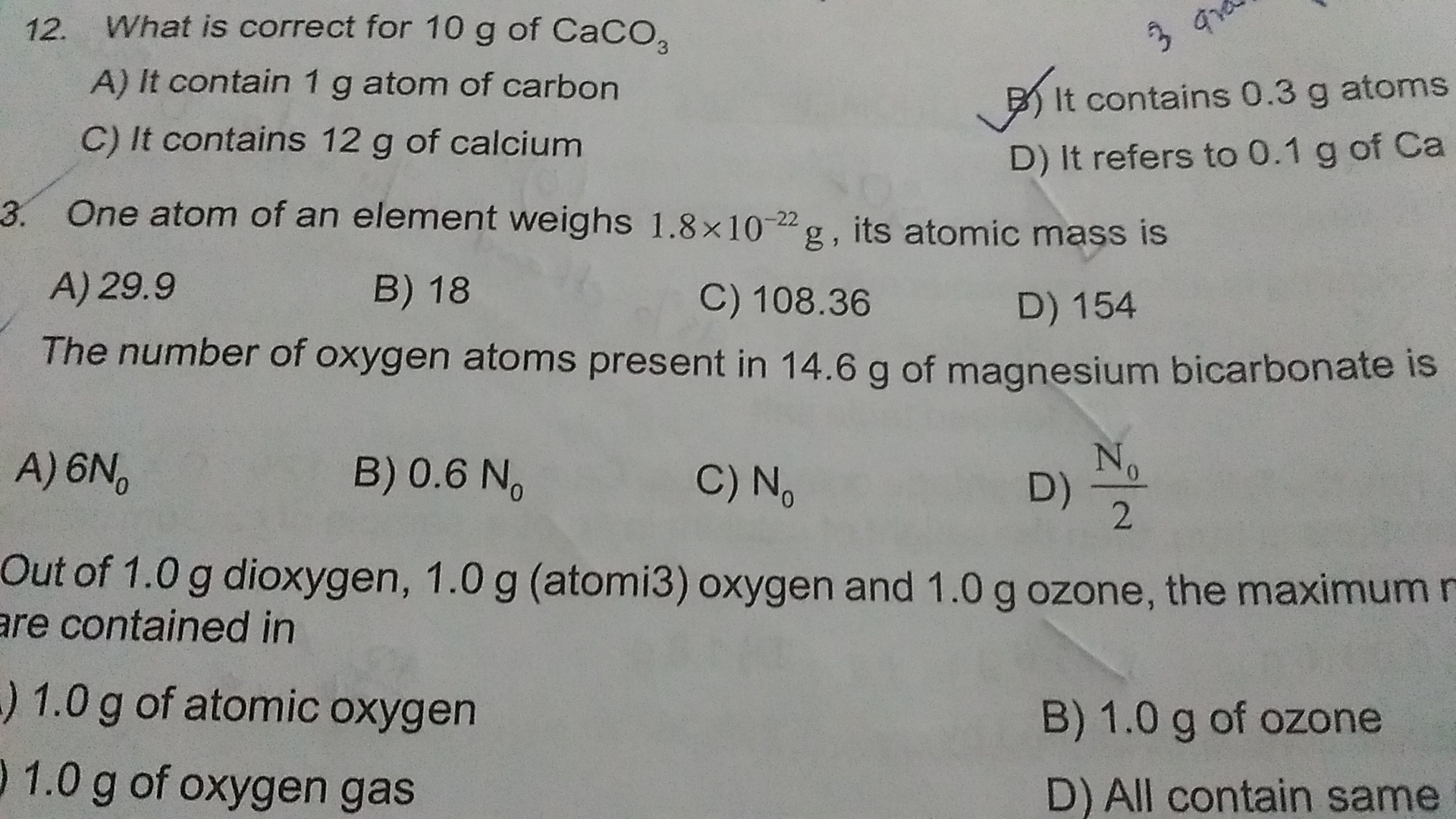
Asked by sildasholly2002 | 29 Jun, 2019, 19:59: PM
Q.no.12
Option B is correct.
Given:
Mass of CaCO3 = 10 gm
Molar mass of CaCO3 = 100 gm
No. of mols of CaCO3 = 0.1 mole
1 mole of CaCO3 contains 1 gm atom of Ca
0.1 mole of CaCO3 contains 0.1 gm atom of Ca
0.1 mole of CaCO3 contains 0.1 gm of atom of C
1 mole of CaCO3 contains 3 gm of O
0.1 mole of CaCO3 contains 0.3 gm of O.
Answered by Varsha | 29 Jun, 2019, 23:44: PM
JEE main - Chemistry
Asked by Mdizhanshaikh | 20 May, 2024, 19:18: PM
JEE main - Chemistry
Asked by manishguptaballia.15 | 11 May, 2024, 15:57: PM
JEE main - Chemistry
Asked by ashwinskrishna2006 | 18 Apr, 2024, 17:37: PM
JEE main - Chemistry
Asked by gmafia618 | 04 Apr, 2024, 20:48: PM
JEE main - Chemistry
Asked by jadhavshivtej256 | 27 Feb, 2024, 18:25: PM
JEE main - Chemistry
Asked by pradumankumarsah1 | 30 Jan, 2024, 14:36: PM
JEE main - Chemistry
Asked by srujan11042008 | 06 Nov, 2023, 10:31: AM





