CBSE Class 11-science Answered
Please explain the molecular orbital theory and also molecular orbital configuration of diatomic molecule.
Asked by GARIMA SRIVASTAVA | 19 Feb, 2011, 10:50: PM
Dear Student
The aim of of molecular orbital theory is to describe the formation of molecules in a similar way as atoms, that is, in terms of orbitals, orbital diagrams, and electron configurations.
So, molecular orbital (MO) theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms.
Theory -- The basic theory of this theory is linear combination of atomic orbitals. The molecular orbitals are formed through the linear combination of atomic orbitals (LCAO).
The molecular orbitals are further divided according to the types of atomic orbitals combining to form a bond. These orbitals are results of electron-nucleus interactions that are caused by the fundamental force of electromagnetism.
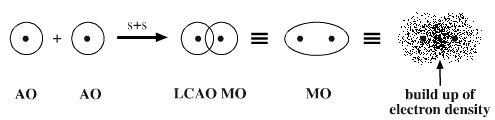
The electrons of the involved atoms are counted and filled in the molecular orbitals in the following order starting from σ 1s orbital. the atomic orbitals of nearly same or same energy overlap and form a bonding and an antibonding molecular orbital. Each of these molecular orbitals consits of a maximum of two electrons. For example the 1s of the two atoms overlap and form σ 1s and σ * 1s. Similarly the px of one atom overlaps with px of the other atom and forms π and π* molecular orbitals.
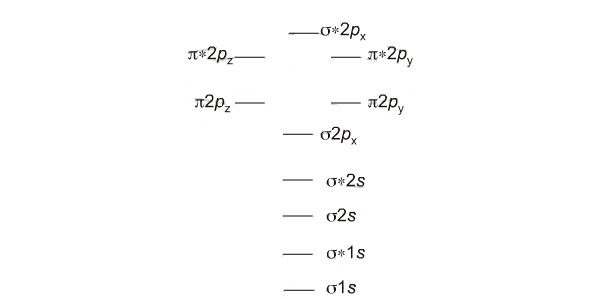
How to write the electronic configuration?
The electronic configuration is written foloowing the same order as we fill the molecular orbitals.
For example Lets take an example of lithium.
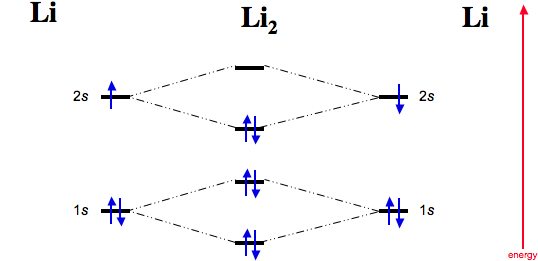
So, we will write the electronic configuration as follows:
σ1s2 σ*1s2, σ2s2
We hope that clarifies your query.
Regards
Team
Topperlearning
Answered by | 20 Feb, 2011, 09:49: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by thesouro007 | 20 Mar, 2024, 06:05: AM
CBSE 11-science - Chemistry
Asked by kamalpavenkp123 | 11 Mar, 2024, 02:49: PM
CBSE 11-science - Chemistry
Asked by Trisha Gupta | 30 Oct, 2022, 05:36: PM
CBSE 11-science - Chemistry
Asked by visalvinod06 | 23 Jun, 2022, 07:39: AM
CBSE 11-science - Chemistry
Asked by bhagwatkrutika6 | 22 Jun, 2022, 09:53: PM
CBSE 11-science - Chemistry
Asked by shabnamaijaz83 | 19 Jun, 2022, 10:08: AM
CBSE 11-science - Chemistry
Asked by akankhyapradhan123 | 16 Jan, 2022, 07:46: AM
CBSE 11-science - Chemistry
Asked by abnarsale | 31 Dec, 2021, 10:41: AM
CBSE 11-science - Chemistry
Asked by naveenbahuguna05 | 11 Dec, 2021, 03:19: PM
CBSE 11-science - Chemistry
Asked by akhileshpandeypandey456 | 12 Aug, 2021, 11:09: PM






