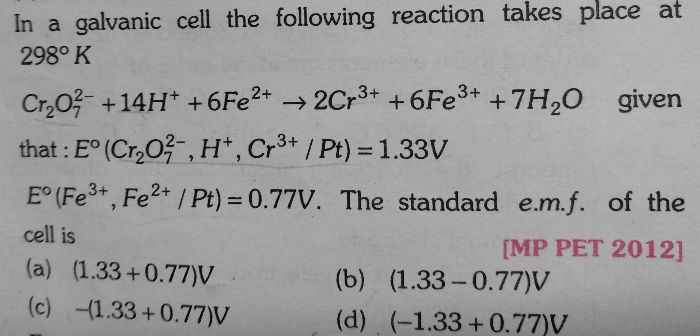NEET Class neet Answered
Please answer the following question.

Asked by Balbir | 09 Sep, 2019, 06:02: PM
Given:
Molar specific conductance = 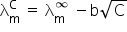
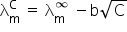
Molar specific conductance at infinite dilution =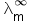
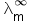
Concentration = C
1. Molar concentration of NaCl at infinite dilution;
C = 4 × 10−4
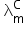 = 107 ohm−1cm2mol−1
= 107 ohm−1cm2mol−1 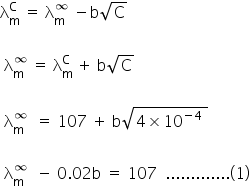
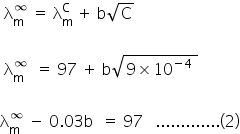
From eq (1) and (2)
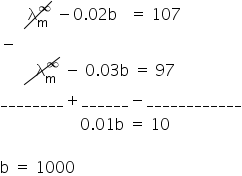
Putting value of b in eq (1)
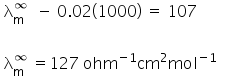
Molar concentration of NaCl at infinite dilution is 127 ohm−1cm2mol−1
2. Cell constant:
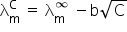
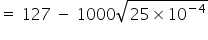
= 127 − 50
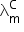 = 77
= 77 We know,
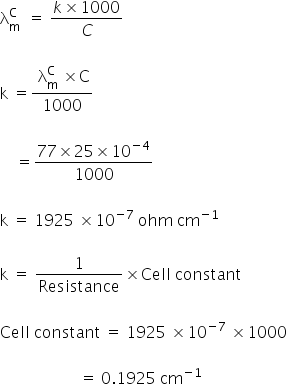
3. Molar conductance:
Given:
C = 5 × 10−3 N
Resistance = 400 ohm
Molar conductance = ?
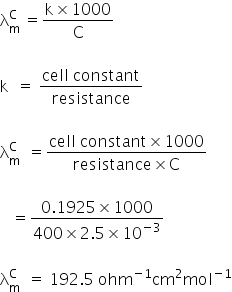
Molar conductance is 192.5 ohm−1cm2mol−1
Answered by Varsha | 10 Sep, 2019, 12:38: PM
NEET neet - Chemistry
Asked by sr8834055 | 20 Mar, 2024, 02:54: PM
NEET neet - Chemistry
Asked by dshreya247 | 03 Feb, 2024, 11:32: AM
NEET neet - Chemistry
Asked by sujitjana971 | 15 Dec, 2022, 08:09: PM
NEET neet - Chemistry
Asked by zoyakhan98264 | 16 Jul, 2022, 01:59: PM
NEET neet - Chemistry
Asked by gurugubellisaivishal2705 | 30 Jun, 2022, 12:32: PM
NEET neet - Chemistry
Asked by ansh.bharso | 28 Jun, 2022, 03:33: PM
NEET neet - Chemistry
Asked by dev28011997 | 09 Oct, 2021, 02:21: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 22 Aug, 2020, 09:43: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 22 Aug, 2020, 09:39: PM