CBSE Class 12-science Answered
Pl answer

Asked by jain.pradeep | 26 Feb, 2020, 09:59: AM
option (b) is correct.
The structure of SO3 molecule in gas phase is Plannar triangular.
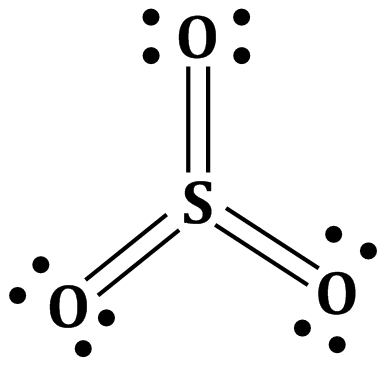
Answered by Varsha | 26 Feb, 2020, 17:20: PM
CBSE 12-science - Chemistry
Asked by navadeepnavadeep242 | 19 Mar, 2024, 20:56: PM
CBSE 12-science - Chemistry
Asked by arjunsah797 | 10 May, 2022, 12:16: PM
CBSE 12-science - Chemistry
Asked by fishtailfever | 21 Feb, 2021, 14:07: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 27 Feb, 2020, 14:50: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 26 Feb, 2020, 09:59: AM
CBSE 12-science - Chemistry
Asked by kasireddyteja15 | 04 Dec, 2019, 21:52: PM
CBSE 12-science - Chemistry
Asked by jaideepsnatu | 21 Feb, 2019, 16:04: PM
CBSE 12-science - Chemistry
Asked by amitarastogijuly12 | 24 Jan, 2019, 16:48: PM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 30 Jul, 2018, 17:33: PM



