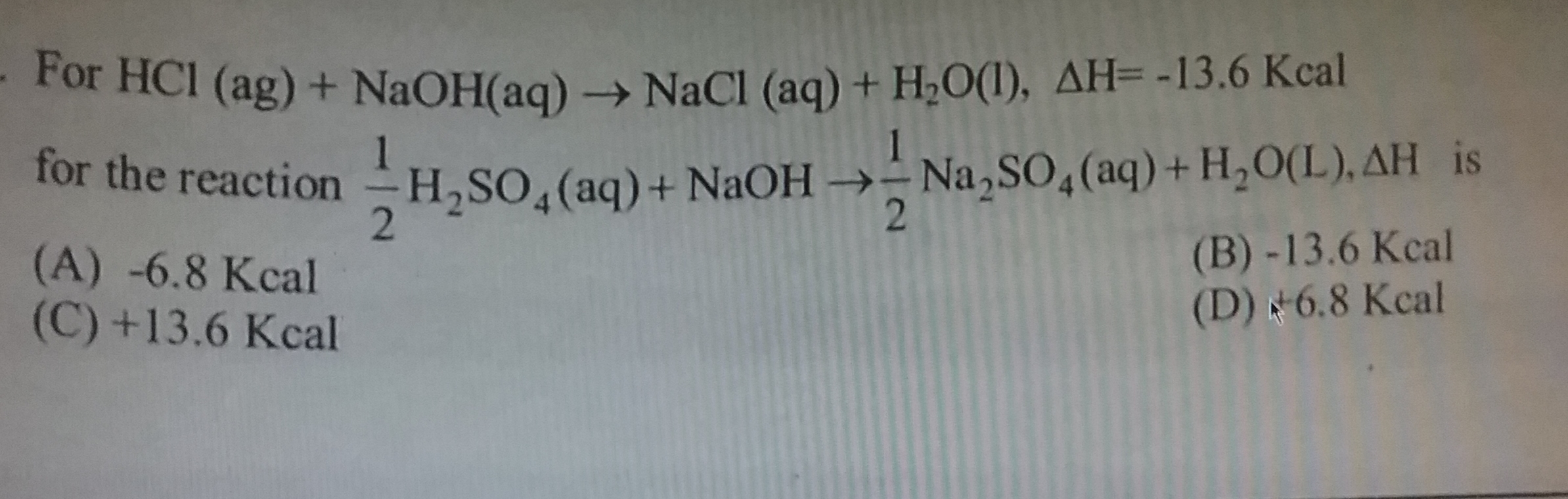CBSE Class 11-science Answered
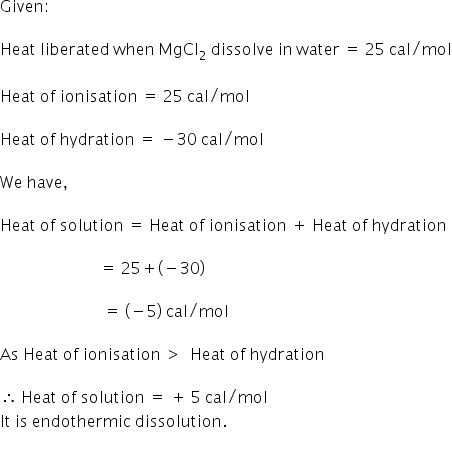
One mole of anhydrous MgCl2 dissolves in water and librates 25 cal/mol of heat. ∆Hhydration of MgCl2 = –30 cal/mol. Heat of dissolution of MgCl2.7H2O(s) is :-
(1)
+5 cal/mol
(2)
–5 cal/mol
(3)
55 cal/mol
(4)
–55 cal/mol
Asked by Atulcaald | 25 May, 2018, 12:24: AM
Option (1) is correct.
Heat of dissolution is + 5 cal/mol.
Solution:
Answered by Varsha | 25 May, 2018, 11:31: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by advssdrall | 11 Jan, 2022, 07:44: PM
CBSE 11-science - Chemistry
Asked by adityasolanki7773 | 22 Oct, 2020, 03:40: PM
CBSE 11-science - Chemistry
Asked by pranavisrihari | 08 Sep, 2020, 05:24: PM
CBSE 11-science - Chemistry
Asked by varakalasuchi3 | 28 Mar, 2020, 04:47: PM
CBSE 11-science - Chemistry
Asked by patra04011965 | 09 Nov, 2019, 12:18: PM
CBSE 11-science - Chemistry
Asked by prakriti12oct | 27 Sep, 2019, 01:43: AM
CBSE 11-science - Chemistry
Asked by prakriti12oct | 26 Sep, 2019, 01:40: AM
CBSE 11-science - Chemistry
Asked by sayantan.chem2 | 06 Aug, 2019, 05:07: PM
CBSE 11-science - Chemistry
Asked by lovemaan5500 | 21 Jan, 2019, 06:37: AM
CBSE 11-science - Chemistry
Asked by Atulcaald | 25 May, 2018, 12:24: AM