ICSE Class 9 Answered
It is found on heating a gas it's volume increases by 50% and pressure decreases to 60% of original value. If the original temperature was -15degree C find the temperature to which it was heated ?
Asked by kanchanrai9794870095 | 02 Dec, 2019, 21:32: PM
We know,
PV/T = constant ...(1)
T1 = -15°C = -15 + 273 K = 258 K, T2 =?
Also, it is given that,
When volume increases by 50%, pressure decreases by 60%
V1 = V, V2 = V + (50/100)V = (3/2) V
P1 = P, P2 = (60/100)P = (3/5) P
Thus,
From equation (1),
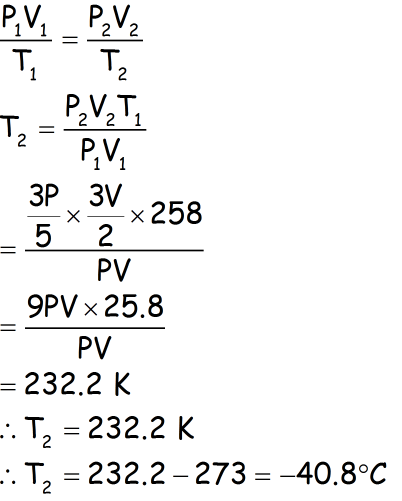
Answered by Shiwani Sawant | 04 Dec, 2019, 15:41: PM
Concept Videos
ICSE 9 - Physics
Asked by thesangeeta1990 | 08 Mar, 2024, 16:37: PM
ICSE 9 - Physics
Asked by lakshyarajsharma94 | 19 Dec, 2021, 12:37: PM
ICSE 9 - Physics
Asked by amit.clw4 | 03 Feb, 2021, 11:30: AM
ICSE 9 - Physics
Asked by kalpanasingh.ito | 29 Jan, 2020, 13:18: PM
ICSE 9 - Physics
Asked by kanchanrai9794870095 | 02 Dec, 2019, 21:32: PM
ICSE 9 - Physics
Asked by dishadistributorslko | 09 Oct, 2019, 23:53: PM
ICSE 9 - Physics
Asked by dharansingh36 | 24 Oct, 2018, 16:56: PM


