CBSE Class 12-science Answered
how zncl2 help in prepration of haloalkanes in groove process?
Asked by gargimoitreyee | 17 Mar, 2018, 11:09: AM
Role of anhydrous ZnCl2:
It helps cleave the C–O bond. Being a Lewis acid, it coordinates with the oxygen
atom of the alcohols. This result in weakening of the C–O bond which ultimately
breaks to form carbocations.
Answered by Varsha | 17 Mar, 2018, 12:30: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by roshanisharma200611 | 07 Feb, 2024, 01:18: PM
CBSE 12-science - Chemistry
Asked by surekhas66675 | 02 Sep, 2021, 05:17: PM
CBSE 12-science - Chemistry
Asked by nazimb0313 | 02 Sep, 2020, 09:34: AM
CBSE 12-science - Chemistry
Asked by mastertask199 | 13 May, 2020, 04:08: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 03 Feb, 2020, 10:42: PM
CBSE 12-science - Chemistry
Asked by ajaysankhala051 | 11 Sep, 2019, 01:19: PM
CBSE 12-science - Chemistry
Asked by pragyachandraul03 | 23 Aug, 2019, 02:12: PM
CBSE 12-science - Chemistry
Asked by priadkonkar | 21 Jan, 2019, 08:52: PM
CBSE 12-science - Chemistry
Asked by rakeshraghav33 | 21 Jan, 2019, 02:49: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 16 May, 2018, 02:34: PM




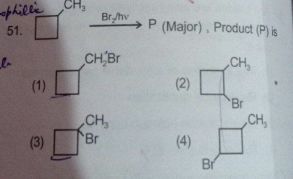
 Total No. of Mono Brominated product :-
Total No. of Mono Brominated product :-