CBSE Class 12-science Answered
how is the cabonyl group of aldehyde/ketone affected in the presence of electron donating group like metyl in cyanohydrin reaction?
Asked by prakriti12oct | 14 Feb, 2020, 12:09: PM
(a) Formation of cyanohydrins:
- On addition of HCN to aldehydes and ketones they yield cyanohydrins.
- Since the reaction is very slow with pure HCN, it is catalysed with the help of a base and the cyanide ion (CN-) generated as a strong nucleophile adds to carbonyl compounds to give cyanohydrins.
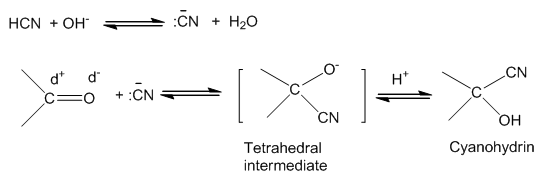
- The C-O double bond is polarised since oxygen is electronegative than carbon.
- So the carbonyl carbon is an electrophilic centre and the carbonyl oxygen is a nucleophilic centre.
- If an electron donating group is present next to carbonyl group then it will slow down the reaction or will retard the rate of reaction.
Answered by Ramandeep | 14 Feb, 2020, 17:57: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by ankitmonda.bankhatil | 11 Jun, 2024, 19:10: PM
CBSE 12-science - Chemistry
Asked by ukg8612 | 15 Apr, 2024, 19:36: PM
CBSE 12-science - Chemistry
Asked by ajayarchi | 08 Feb, 2024, 03:43: AM
CBSE 12-science - Chemistry
Asked by pallasriramulu9 | 24 Dec, 2023, 06:05: AM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 30 Jun, 2021, 16:52: PM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 28 Jun, 2021, 14:34: PM
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 31 Dec, 2020, 10:45: AM
CBSE 12-science - Chemistry
Asked by shreevarshni1910 | 16 Jun, 2020, 13:36: PM
CBSE 12-science - Chemistry
Asked by yukthas706 | 11 Jun, 2020, 10:00: AM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 14 Feb, 2020, 12:09: PM







