CBSE Class 12-science Answered
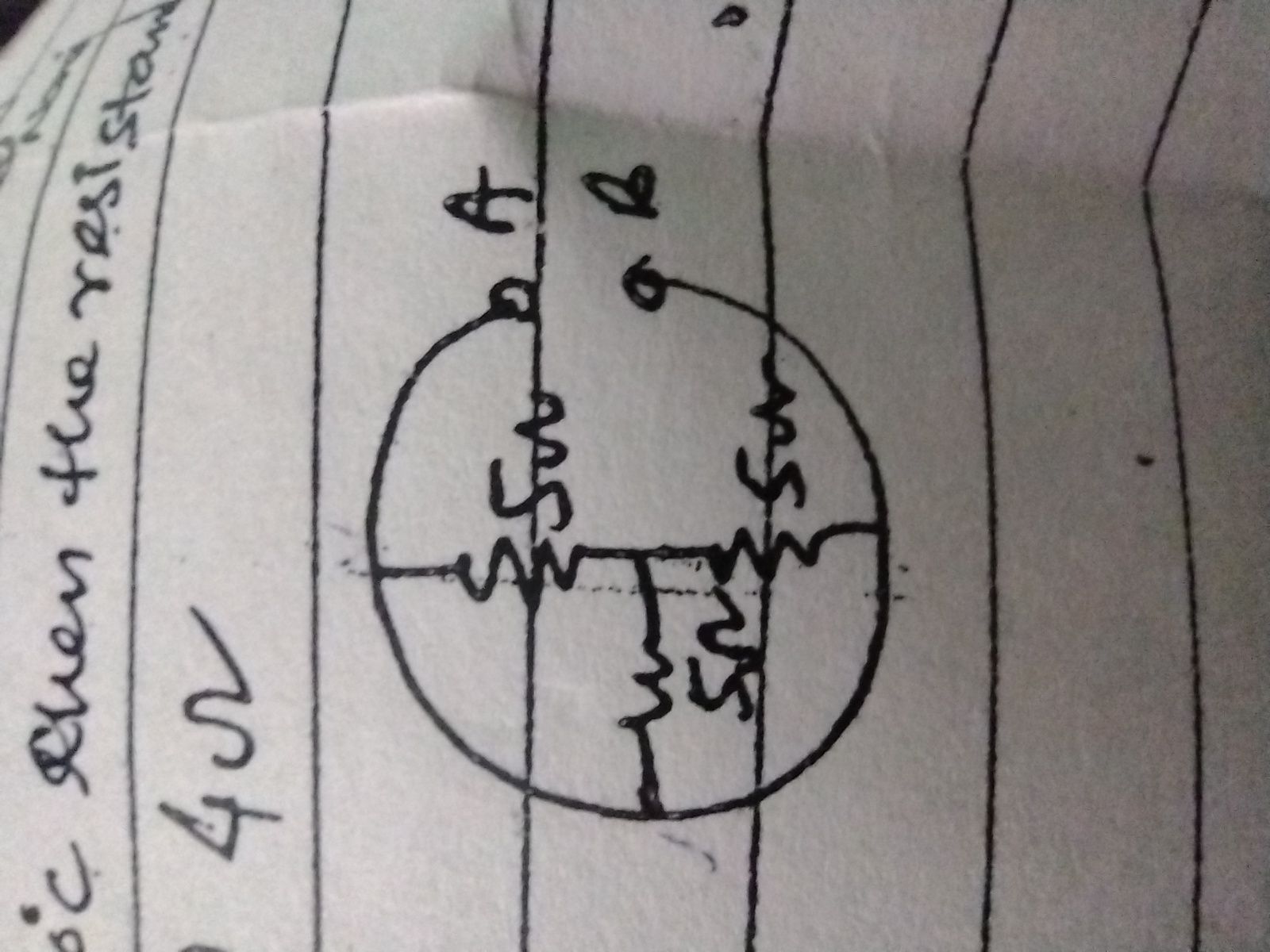
How is electrolytic conduction different from metallic conduction ?
Asked by bhanu_kumar | 08 Aug, 2009, 08:24: AM
Metallic conduction is due to availability of free electrons in the metal which move when an electric potential difference is applied across the terminals of metal conductor.
In electrolytic conduction, it's the movement of ions, and that's the main difference.
Regards,
Team,
TopperLearning.
Answered by | 08 Aug, 2009, 01:48: PM
Concept Videos
CBSE 12-science - Physics
Asked by mailtoanjalip2005 | 16 Mar, 2024, 08:22: PM
CBSE 12-science - Physics
Asked by patelnamra608 | 26 Jan, 2024, 11:01: AM
CBSE 12-science - Physics
Asked by smitdholakiya28 | 17 Dec, 2023, 09:37: AM
CBSE 12-science - Physics
Asked by shivashikhar69 | 09 Dec, 2023, 02:27: PM
CBSE 12-science - Physics
Asked by nikhilsai2616 | 19 Nov, 2023, 01:05: AM
CBSE 12-science - Physics
Asked by snehashiragannavar773 | 21 Oct, 2023, 02:38: PM
CBSE 12-science - Physics
Asked by dr.strange45678 | 02 Aug, 2023, 12:31: PM
CBSE 12-science - Physics
Asked by komalbrar0987 | 17 May, 2023, 06:15: PM
CBSE 12-science - Physics
Asked by amanpanday6384 | 22 Dec, 2022, 12:42: PM