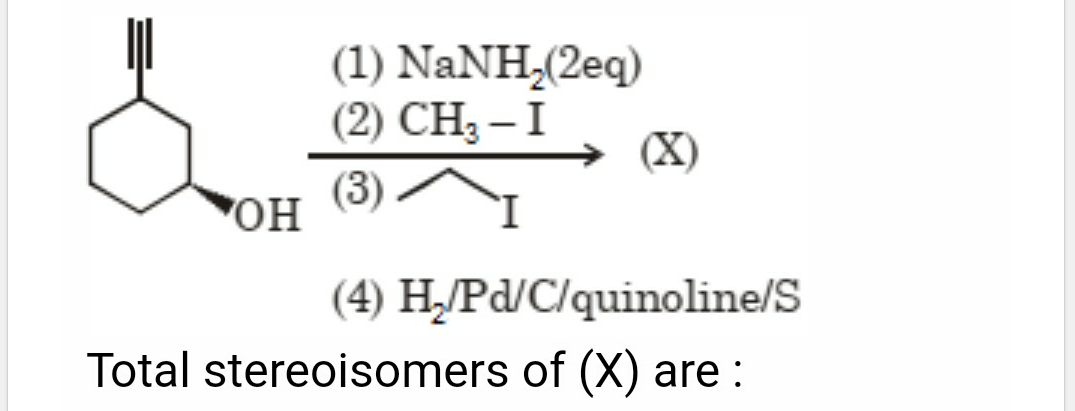JEE Class main Answered
how do i calculate the strereoisomers ?
Asked by shanmugamneet2018 | 28 Mar, 2019, 10:19: AM
Case I: When the molecule has no symmetry (usymmetrical):
The number of d- and l- forms, a = 2n , where n= number of unsymmetrical carbon atoms.
number of meso isomers m = 0
The total number of optical isomers = a + m = 2n
For example, 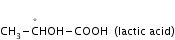
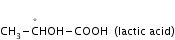
in the above compound n= 1, a = 21 = 2, m= 0,
hence total optical isomers = 2 + 0 = 2
Case II: When the molecule has symmetry:
1) When the number of asymmetric carbon atoms are even:
The number of d- and l- forms, a = 2(n-1) , where n= number of unsymmetrical carbon atoms.
number of meso isomers m = 2(n/2)-1 ,
The total number of optical isomers = a + m = 2(n-1) +2(n/2)-1
For example,
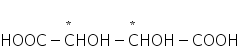
where n=2, a=2(n-1) = 2(2-1) = 21 = 2
m = 2(n/2)-1 = 2(2/2)-1 = 20 = 1
So, total optical isomers = 2+1 = 3
2) When the number of asymmetric carbon atoms are odd:
The number of d- and l- forms, a = 2(n-1) - 2(n-1)/2 where n= number of unsymmetrical carbon atoms.
number of meso isomers m = 2(n-1)/2
The total number of optical isomers = a + m = 2(n-1)
For example,
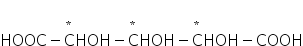
where n=3, a = 2(n-1) - 2(n-1)/2 = 22 - 21 = 2
m = 2(n-1)/2 = 2(3-1)-2 = 21 = 2
So, total optical isomers = 2+2 = 4
In all above three cases, the number of racemic forms will be = a/2
We hope you understand the concept, still, you have any query you can ask in doubts and solution section.
Answered by Ramandeep | 28 Mar, 2019, 11:24: AM
JEE main - Chemistry
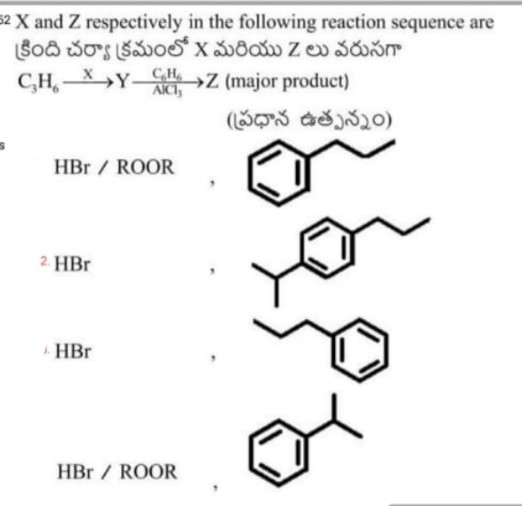
Asked by neerajavuppala1983 | 23 Jul, 2024, 22:49: PM
JEE main - Chemistry
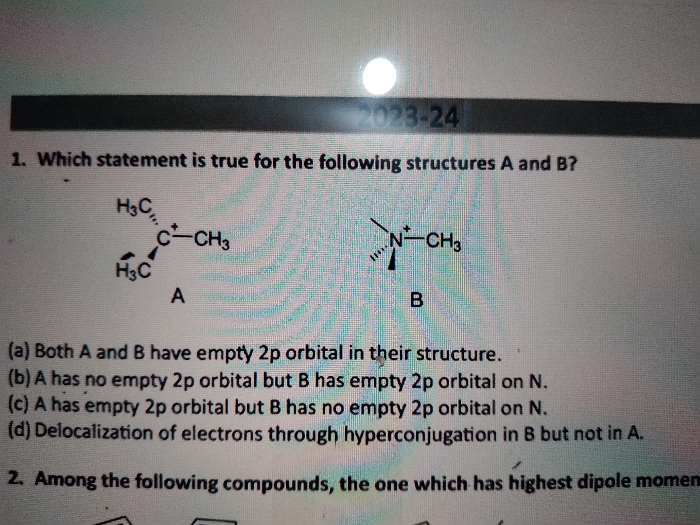
Asked by amanniju2 | 15 May, 2024, 09:05: AM
JEE main - Chemistry
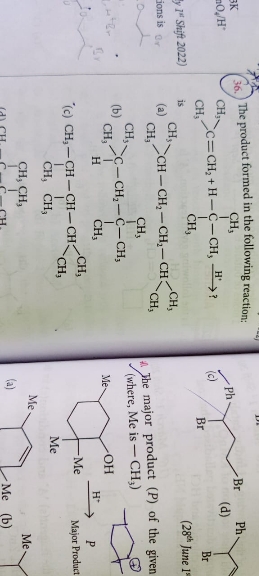
Asked by srkgb8018 | 10 May, 2024, 07:36: AM
JEE main - Chemistry
Asked by jacksparrow7352133590 | 09 May, 2024, 20:20: PM
JEE main - Chemistry
Asked by patelamrutbhaib24 | 28 Jan, 2024, 13:44: PM
JEE main - Chemistry
Asked by harshakancharla329 | 27 Jan, 2024, 09:07: AM
JEE main - Chemistry
Asked by mp797056 | 23 Oct, 2023, 16:23: PM
JEE main - Chemistry
Asked by dheerajrao2005 | 26 Mar, 2022, 19:06: PM
JEE main - Chemistry
Asked by yasharthshankar | 05 Jun, 2020, 23:39: PM