ICSE Class 9 Answered
find the weight of sodium nitrate required to prepare 60g pure crystals from its saturated solution at 70c.solubility of sodium nitrate is 140g at 70c and 100 at 25c.("c"stands for celsius of temperature
Asked by dishaprep | 01 Sep, 2018, 23:03: PM
Given:
Solubility at 70°C = 140
Solubility at 70°C = 100
Amount of crystals obtained when the solution is cooled from 70°C to 25°C
= 140-100 = 40 g
To obtain 40 g of crystals sodium nitrate is taken is 140 g.
To obtain 60 g crystals, sodium nitrate required will be = 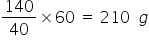
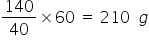
Answered by Ramandeep | 03 Sep, 2018, 12:30: PM
Application Videos
Concept Videos
ICSE 9 - Chemistry
Asked by shreesamaira | 08 Aug, 2021, 11:48: AM
ICSE 9 - Chemistry
Asked by khatripunit39 | 31 Jul, 2021, 13:41: PM
ICSE 9 - Chemistry
Asked by screen1974 | 10 Jul, 2019, 20:21: PM
ICSE 9 - Chemistry
Asked by sugandhpatna | 24 Oct, 2018, 12:33: PM
ICSE 9 - Chemistry
Asked by dishaprep | 01 Sep, 2018, 23:03: PM
ICSE 9 - Chemistry
Asked by Kanwaranita10 | 12 May, 2018, 08:00: AM
ICSE 9 - Chemistry
Asked by sr.edits007 | 13 Feb, 2018, 08:35: AM





