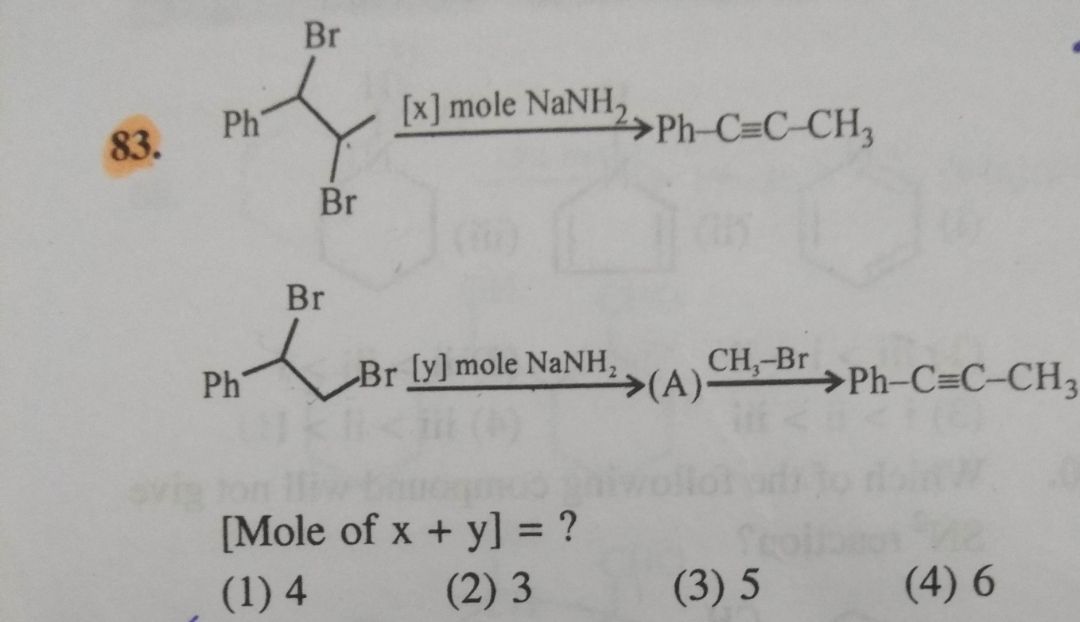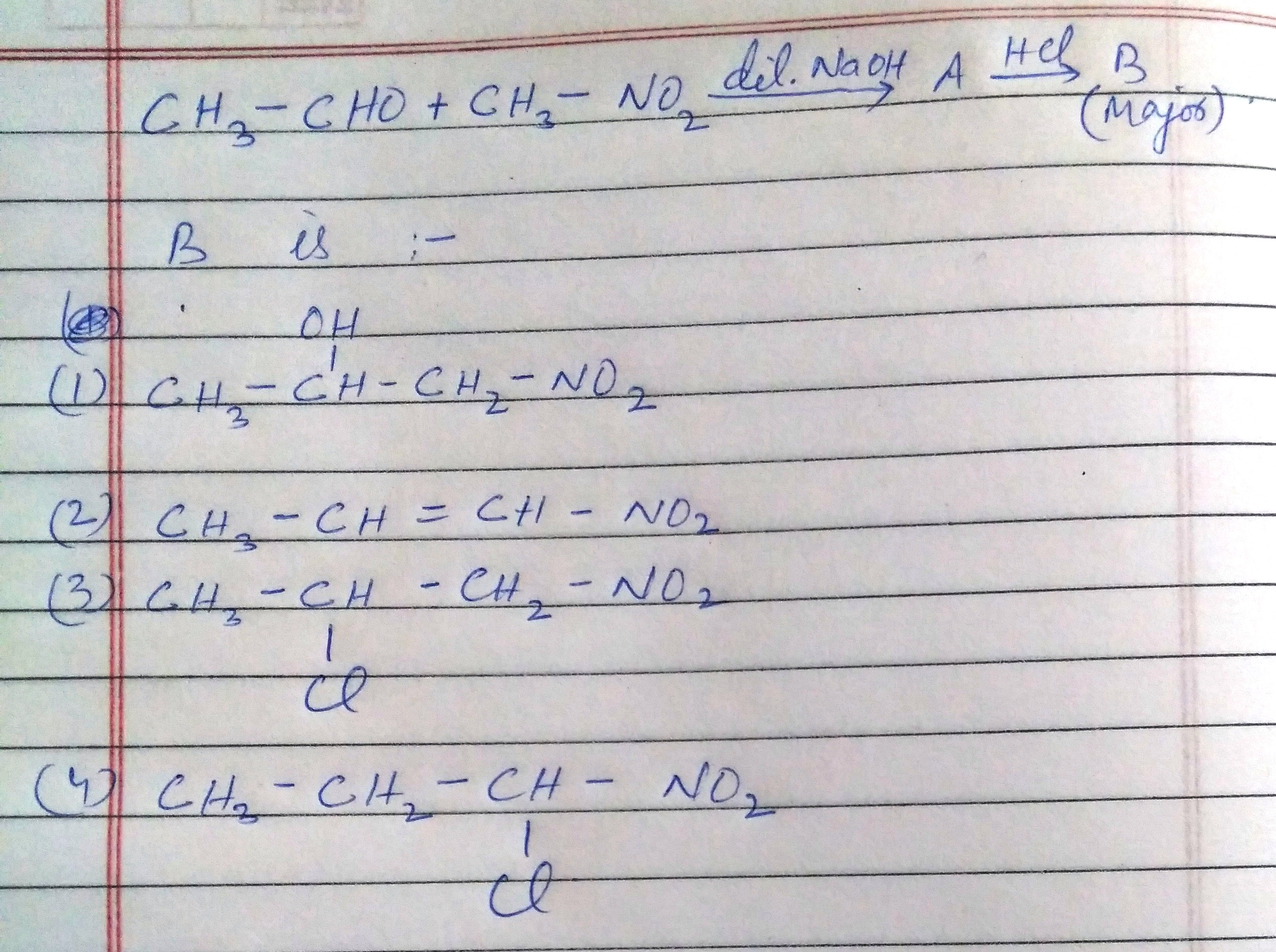NEET Class neet Answered
⭕ Explain why product formed at meta position is more stable then para position ?
Also explain the mechanism... thanks ☺️

Asked by jhajuhi19 | 13 Apr, 2020, 08:27: AM
p-Chlorotoluene first gives benzyne with methyl as substituent. When -NH2 attacks at carbon which is bearing triple bond it gives mixture of meta and para-toluidine. Major product is m-toluidine because carbanion formed at meta position by attack of nucleophile is more stable comapre to para position.
Answered by Ramandeep | 13 Apr, 2020, 01:49: PM
NEET neet - Chemistry
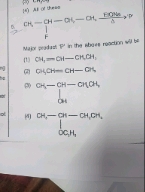
Asked by hk62929363 | 04 Mar, 2024, 03:56: AM
NEET neet - Chemistry
Asked by samramojuru | 22 Feb, 2024, 04:18: PM
NEET neet - Chemistry
Asked by swetadayal036 | 15 Jan, 2024, 08:04: PM
NEET neet - Chemistry
Asked by sabhachoudhary0786 | 15 Sep, 2023, 04:53: PM
NEET neet - Chemistry
Asked by jhajuhi19 | 05 Sep, 2020, 05:35: AM
NEET neet - Chemistry
Asked by jhajuhi19 | 11 Aug, 2020, 04:25: AM
NEET neet - Chemistry
Asked by subhasanth | 29 Apr, 2020, 10:53: AM
NEET neet - Chemistry
Asked by jhajuhi19 | 13 Apr, 2020, 08:27: AM
NEET neet - Chemistry
Asked by harunabbas | 08 Apr, 2020, 02:13: PM
NEET neet - Chemistry
Asked by prakriti12oct | 30 Mar, 2020, 01:23: AM