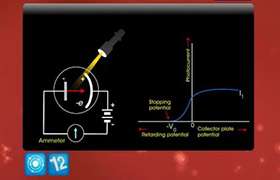
CBSE Class 12-science Answered
dual
Asked by chandant | 01 Oct, 2010, 05:00: PM
Dear Student
For any hydrogen like element:

where
 is the wavelength of the light emitted in vacuum;
is the wavelength of the light emitted in vacuum;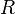 is the Rydberg constant for this element;
is the Rydberg constant for this element; is the atomic number, i.e. the number of protons in the atomic nucleus of this element;
is the atomic number, i.e. the number of protons in the atomic nucleus of this element; and
and  are integers such that
are integers such that  .
.
For hydrogen, Z = 1
For Helium, Z = 2
Putting in the above expression:
λhelium = λhydrogen / 4 (for same transition) = 4860/4 = 1215 angstrom
We hope that satisfies your query.
Regards
Team
Topperlearning
Answered by | 01 Oct, 2010, 10:33: PM
Concept Videos
CBSE 12-science - Physics
Asked by mishrigupta19319 | 08 Apr, 2024, 06:28: PM
CBSE 12-science - Physics
Asked by mishrigupta19319 | 07 Apr, 2024, 11:23: AM
CBSE 12-science - Physics
Asked by mazhartahsildar143 | 07 May, 2020, 01:44: PM
CBSE 12-science - Physics
Asked by Hemanthrakshitha95 | 03 Mar, 2020, 02:55: PM
CBSE 12-science - Physics
Asked by shivakumarshreyas24 | 01 Mar, 2020, 08:12: AM
CBSE 12-science - Physics
Asked by khushimassey437 | 31 May, 2019, 08:41: AM
CBSE 12-science - Physics
Asked by jain.pradeep | 26 May, 2019, 06:42: PM
CBSE 12-science - Physics
Asked by manasvijha | 19 Mar, 2019, 07:17: PM
CBSE 12-science - Physics
Asked by rohitraman1115 | 08 Jan, 2019, 04:33: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 21 May, 2014, 09:18: AM