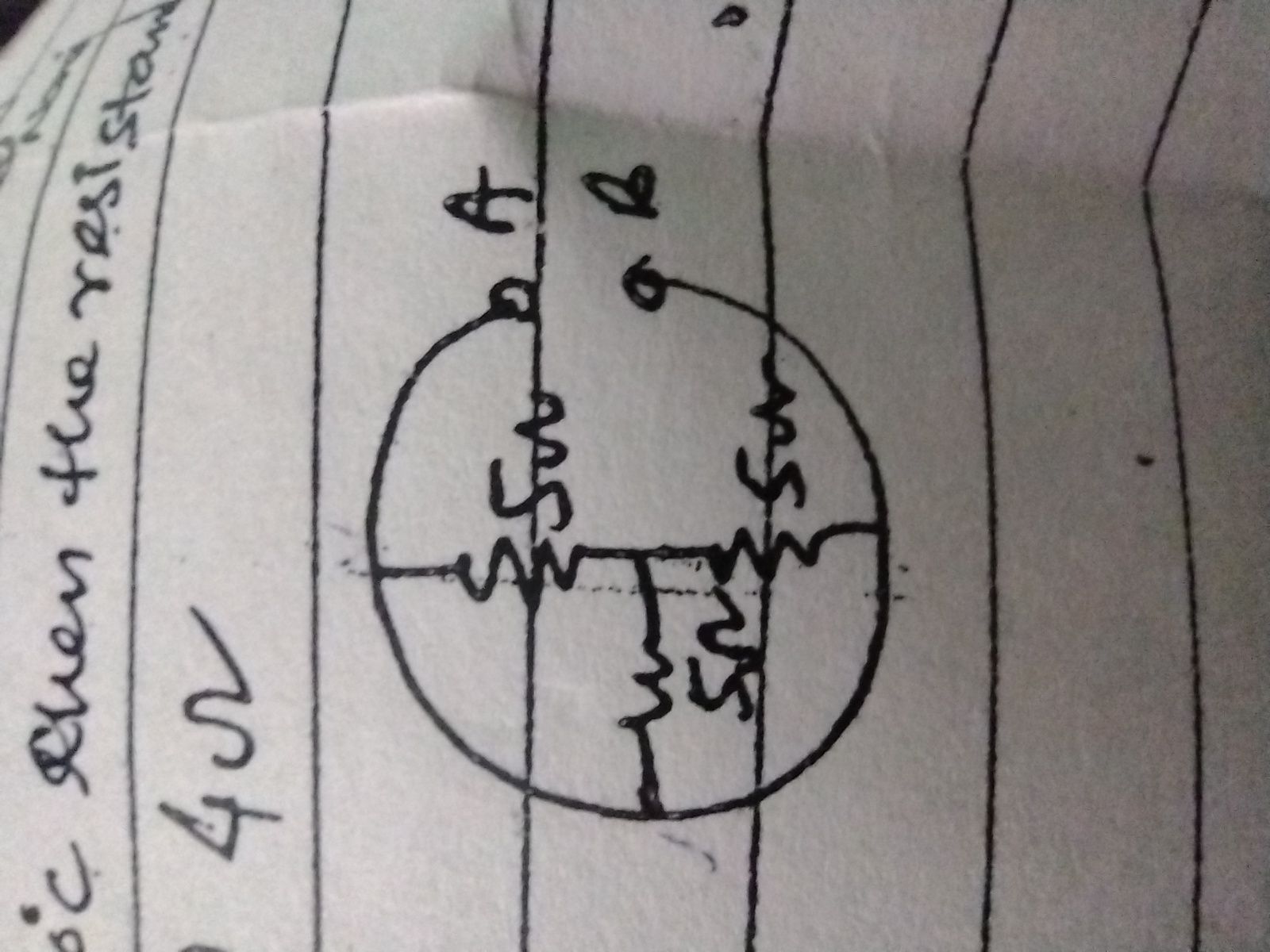
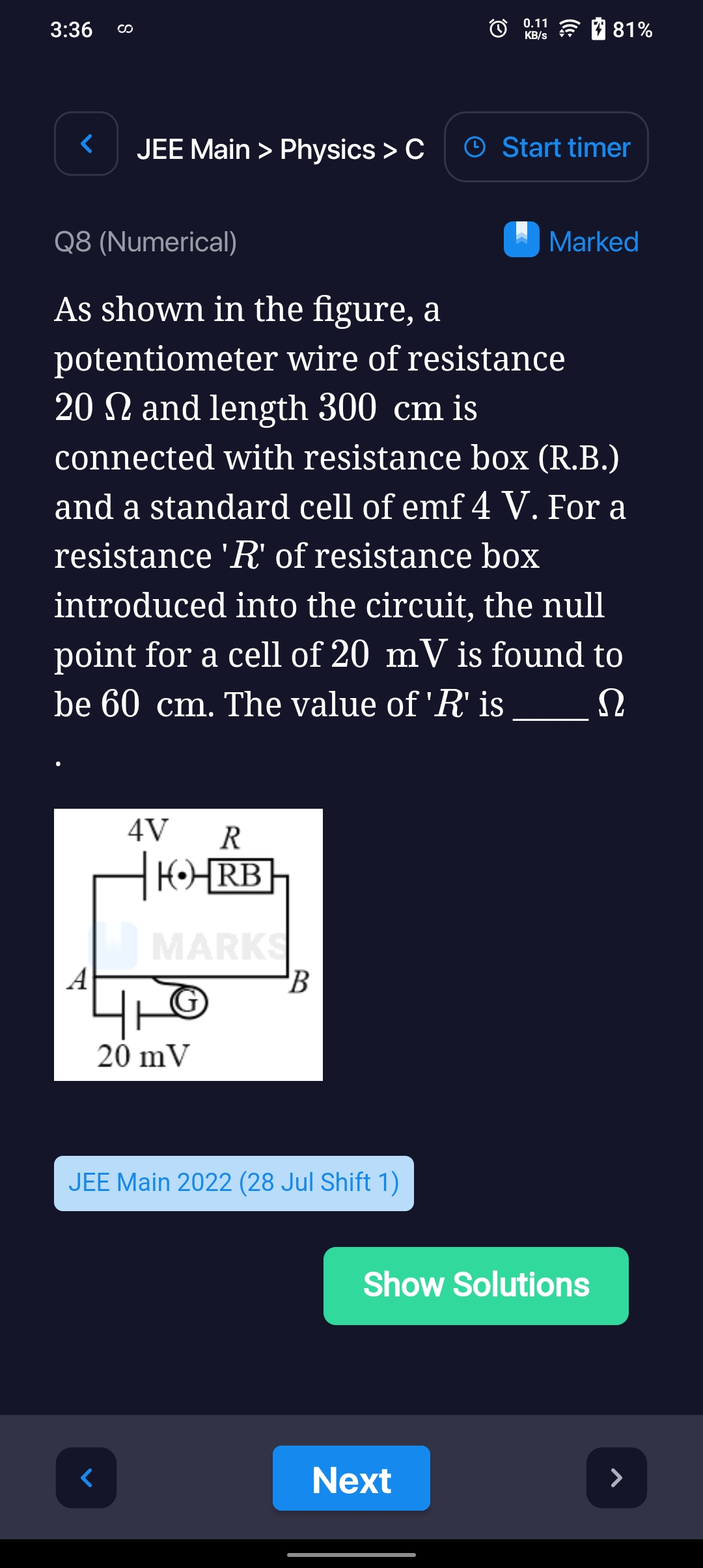
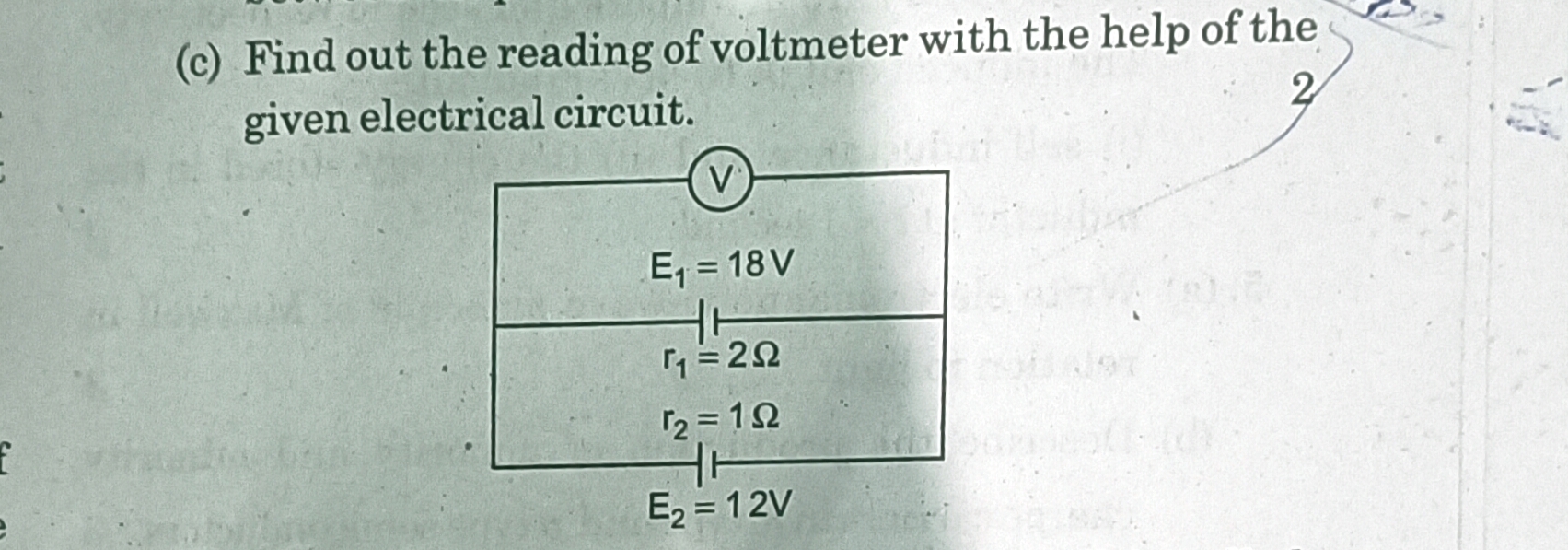
CBSE Class 12-science Answered
In other words, they are electrochemical cells in which the electrochemical reaction that releases energy is readily reversible. Rechargeable electrochemical cells are therefore a type of accumulator. Primary cell is any kind of electrochemical cell in which the electrochemical reaction of interest is not reversible, so used in disposable batteries. Unlike a secondary cell, attempting to reverse the reaction in a primary cell via recharging is dangerous and can lead to a battery explosion. A related difference is that primary batteries use up the materials in one or both of their electrodes, while, ideally, the reversibility of the reactions in a secondary cell allows them to be restored to almost the same fully charged condition on each recharging.