JEE Class main Answered
Detailed solution please.
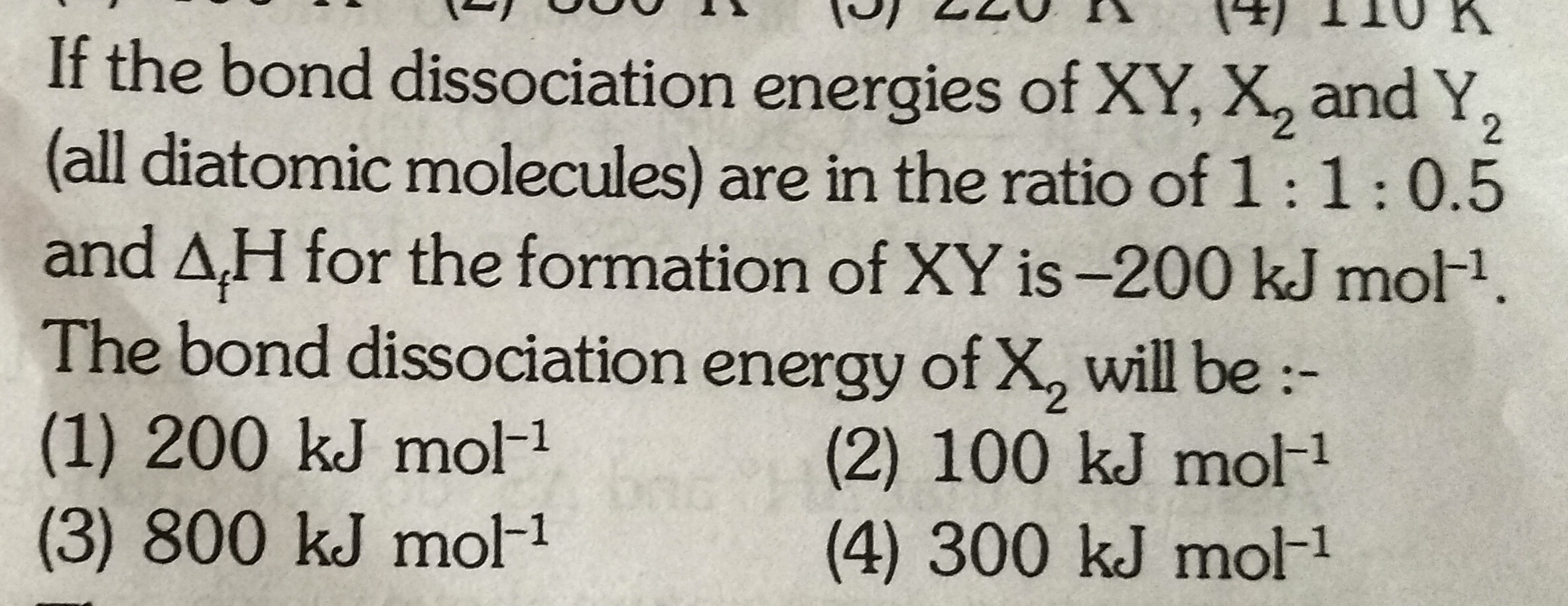
Asked by g_archanasharma | 08 Feb, 2019, 17:49: PM
let the bond dissociation energy of,
X2= x kJ/mol
XY=x kJ/mol
Y2=0.5x kJ/mol
We have,
1/2 X2 + 1/2Y2 → XY ΔHf = -200 kJ/mol
We know that,
ΔHreaction = (bond dissociation energy of reactants) - ( bond dissociation energy of products)
= 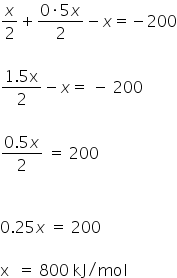
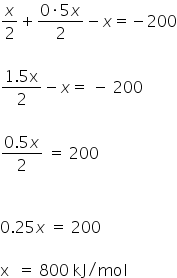
Bond dissociation energy of X2 is 800 kJ/mol.
Answered by Varsha | 11 Feb, 2019, 00:11: AM
JEE main - Chemistry
Asked by mp0985797 | 01 Feb, 2022, 20:38: PM
JEE main - Chemistry
Asked by sgawade2310 | 26 Jun, 2021, 14:58: PM
JEE main - Chemistry
Asked by shrutigandha07 | 15 Apr, 2019, 20:40: PM
JEE main - Chemistry
Asked by vishakhachandan026 | 15 Apr, 2019, 11:34: AM
JEE main - Chemistry
Asked by g_archanasharma | 20 Feb, 2019, 18:04: PM
JEE main - Chemistry
Asked by g_archanasharma | 08 Feb, 2019, 17:49: PM




