JEE Class main Answered
Detailed answers please

Asked by g_archanasharma | 20 Feb, 2019, 18:04: PM
The hydrogenation of cyclohexene:
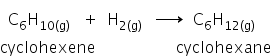
let's find out the ΔHHydrogenation =?
We have given,
(i) 

(ii) 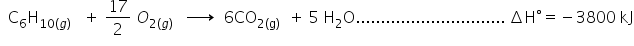
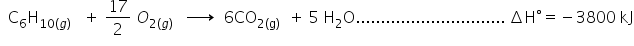
(iii) 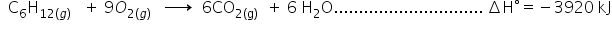
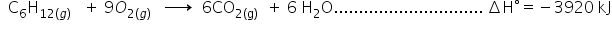
ΔHHydrogenation = (i) + (ii) - (iii)
ΔH° = -241 kJ + (-3800 kJ) - (-3920 kJ)
= - 121 kJ
Answered by Ramandeep | 21 Feb, 2019, 11:07: AM
JEE main - Chemistry
Asked by mp0985797 | 01 Feb, 2022, 20:38: PM
JEE main - Chemistry
Asked by sgawade2310 | 26 Jun, 2021, 14:58: PM
JEE main - Chemistry
Asked by shrutigandha07 | 15 Apr, 2019, 20:40: PM
JEE main - Chemistry
Asked by vishakhachandan026 | 15 Apr, 2019, 11:34: AM
JEE main - Chemistry
Asked by g_archanasharma | 20 Feb, 2019, 18:04: PM
JEE main - Chemistry
Asked by g_archanasharma | 08 Feb, 2019, 17:49: PM