CBSE Class 10 Answered
Dear experts,
Kindly explain the question with a proper solution.
Thank you.

Asked by pratikshyadashrkl | 29 Mar, 2020, 22:08: PM
In the gas mixture , since N2 and He are in equal molar ratio, their partial pressures are alsoequal.
Partial pressure of N2 = 2000 mm of mercury ; Partial pressure of He = 2000 mm of mercury;
As per the given decrease of pressure of N2 with respect to time,
N2 partial pressure will comedown to 500 mm of mercury in 1 hour.
By Graham's law of effusion, Rate of effusion is inversely prportional to square root of molar mass.
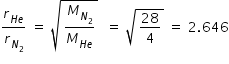
Being a lighter molecule, He will effuse 2.646 times faster than N2 .
Hence pressure of He decreases 2.646 times more compare to Nitrogen.
Hence partial pressure of He after 1 hour = 500 /2.646 = 189 mm of mercury
molar ratio of ( N2 /He ) = 500/189 = 2.646 /1
Answered by Thiyagarajan K | 30 Mar, 2020, 14:41: PM
Application Videos
Concept Videos
CBSE 10 - Physics
Asked by ramkrushnaramkrushna1 | 07 Jul, 2024, 19:01: PM
CBSE 10 - Physics
Asked by mailtoparvathyprajith | 01 Jul, 2024, 13:16: PM
CBSE 10 - Physics
Asked by gs6070260 | 15 Jun, 2024, 20:45: PM
CBSE 10 - Physics
Asked by rv5238428 | 15 Jun, 2024, 19:43: PM
CBSE 10 - Physics
Asked by diljotsingh68641 | 08 Jun, 2024, 19:03: PM
CBSE 10 - Physics
Asked by jainvandan708 | 07 Jun, 2024, 12:06: PM
CBSE 10 - Physics
Asked by kk7629948076 | 03 Jun, 2024, 23:17: PM
CBSE 10 - Physics
Asked by agankitgupta938 | 18 Apr, 2024, 16:29: PM
CBSE 10 - Physics
Asked by infinityupgraded | 13 Apr, 2024, 08:17: AM