CBSE Class 11-science Answered
Commercially available conc. HCl contains 38% HCl by mass.
a) What is the molarity of this solution, if the density is 1.10g/ml?
b) What volume of conc. HCl is required to make 1.0 L of 0.10M HCl?
Asked by Akanksha Srikanth | 07 Dec, 2015, 06:30: PM
Hi,
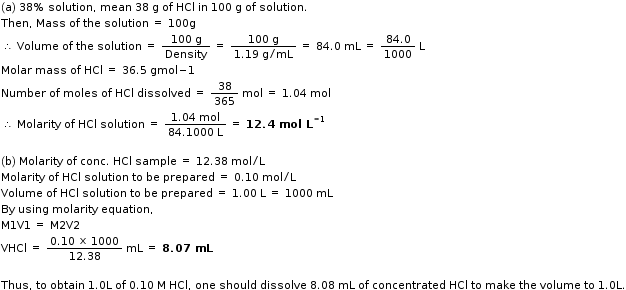
Hope this helps!!
Answered by | 07 Dec, 2015, 07:00: PM
Application Videos
Concept Videos
CBSE 11-science - Chemistry
Asked by gklakshmi701 | 27 Apr, 2024, 09:36: AM
CBSE 11-science - Chemistry
Asked by saranyachakraborty2007 | 25 Apr, 2024, 05:23: AM
CBSE 11-science - Chemistry
Asked by rhythmdraco42 | 22 Apr, 2024, 10:43: PM
CBSE 11-science - Chemistry
Asked by hm6561889 | 15 Apr, 2024, 07:45: AM
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by ansh.skulkarni1158 | 07 Apr, 2024, 11:03: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by josephineanto1960 | 28 Mar, 2024, 12:50: PM













