CBSE Class 11-science Answered
calculate the number of hydrogen atoms and oxygen atom in 1 mole of water!
Asked by bhagwatkrutika6 | 22 Jun, 2022, 20:09: PM
one mole of water contains 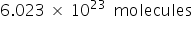 .
.
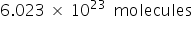 .
.
Answered by Ravi | 27 Jun, 2022, 19:09: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by ajjubhaikhgg851205khgg | 28 May, 2024, 13:30: PM
CBSE 11-science - Chemistry
Asked by anithaanu629940 | 21 May, 2024, 10:12: AM
CBSE 11-science - Chemistry
Asked by r84314179 | 10 May, 2024, 09:04: AM
CBSE 11-science - Chemistry
Asked by r84314179 | 06 May, 2024, 14:28: PM
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 20:15: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 23:04: PM
CBSE 11-science - Chemistry
Asked by avijotsingh946431 | 22 Feb, 2024, 17:36: PM
CBSE 11-science - Chemistry
Asked by gurmelsinghray | 21 Feb, 2024, 08:43: AM
CBSE 11-science - Chemistry
Asked by bablipanwar893 | 01 Jul, 2023, 12:25: PM





