CBSE Class 12-science Answered
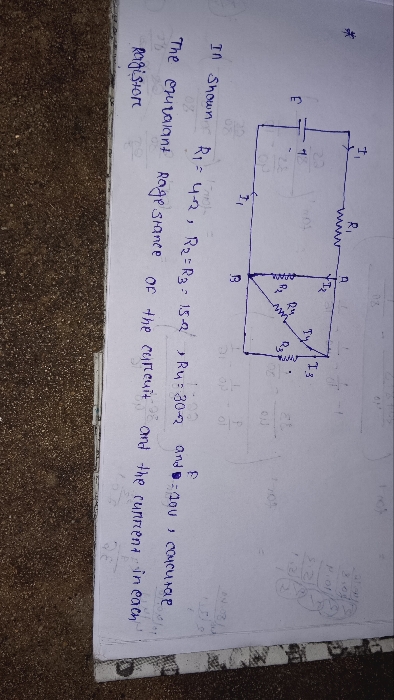
An electron is constrained in one dimensional box of side 0.1nm. Obtain the first four eigen values in electron volt.
Asked by sahupramod650 | 01 Dec, 2019, 02:47: PM
Eigen values of particle confined in one dimensional box is the values of energy levels of different states
designated by quantum number n
Energy level of nth state = En = [ h2 / ( 8 m L2 ) ] n2
where h = 6.626 × 10-34 J , planck's constant
m = 9.109 × 10-31 kg , electron mass
L = 10-10 m = dimension of box
n is quantum state
By substituting the values , we get, En = 6.025 × 10-18 n2 J
since 1 eV = 1.602 × 10-19 J , we express energy of states in eV as, En = (6.025 × 10-18 n2 ) / ( 1.602 × 10-19 ) eV
En = 37.61 n2 eV
Hence , E1 = 37.61 eV
E2 = 37.61 × 4 = 150.44 eV
E3 = 37.61 × 9 = 338.5 eV
E4 = 37.61 × 16 = 601.76 eV
Answered by Thiyagarajan K | 01 Dec, 2019, 04:52: PM
Concept Videos
CBSE 12-science - Physics
Asked by ankush76728 | 06 May, 2024, 04:52: PM
CBSE 12-science - Physics
Asked by ankush76728 | 05 May, 2024, 09:55: PM
CBSE 12-science - Physics
Asked by heymindurownbusiness | 04 May, 2024, 11:15: AM
CBSE 12-science - Physics
Asked by talulu | 01 May, 2024, 05:14: PM
CBSE 12-science - Physics
Asked by kanishkg511 | 30 Apr, 2024, 07:25: PM
CBSE 12-science - Physics
Asked by sahoobanita89 | 30 Apr, 2024, 05:10: AM
CBSE 12-science - Physics
Asked by divakar.9124 | 27 Apr, 2024, 10:42: PM
CBSE 12-science - Physics
Asked by panneer1766 | 24 Apr, 2024, 01:52: PM