JEE Class main Answered
A swimmer coming out from a pool with a film of water weighing about 18 g .how much heat must be supplied to evapourite this water?also calculaye internal energy of vapiurisation at 100? given Enthalpy of vapiurisation at 373k is 40.66kj/mole
Asked by ashutosharnold1998 | 11 Aug, 2019, 00:37: AM
Given:
Mass of water = 18 gm
ΔvapH2O = 40.66 kJ/mol
R = 8.314×10−3 kJ/mol
T = 373 K
Evaporation of water is given as,
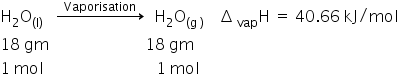
ΔvapU = ΔH − PΔV
=ΔH −ΔngRT
Δng = 1−0
= 1 mol
Therefore
ΔvapU = 40.66 − (1)(8.314×10−3)(373)
= 40.66 − 3.10
ΔvapU = 37.55 kJ/mol
Internal energy of vaporisation is 37.55 kJ/mol
Answered by Varsha | 12 Aug, 2019, 11:19: AM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by mp0985797 | 01 Feb, 2022, 20:38: PM
JEE main - Chemistry
Asked by sgawade2310 | 26 Jun, 2021, 14:58: PM
JEE main - Chemistry
Asked by ashutosharnold1998 | 03 Nov, 2019, 20:22: PM
JEE main - Chemistry
Asked by ashutosharnold1998 | 31 Oct, 2019, 19:16: PM
JEE main - Chemistry
Asked by ashutosharnold1998 | 11 Aug, 2019, 00:37: AM
JEE main - Chemistry
Asked by ashutosharnold1998 | 10 Aug, 2019, 00:10: AM
JEE main - Chemistry
Asked by Ranjeetgupta26068 | 19 May, 2019, 22:14: PM
JEE main - Chemistry
Asked by shrutigandha07 | 15 Apr, 2019, 20:40: PM
JEE main - Chemistry
Asked by vishakhachandan026 | 15 Apr, 2019, 11:34: AM