ICSE Class 9 Answered
A gas is collected at a pressure of 95 cm of Hg and a temperature of 50 degree centegrade.To what temperature should it be cooled so that it occupies a volume which is 80% of its original volume when the pressure of the gas is 90 cm of Hg.
Asked by Pradeep | 31 Oct, 2017, 08:20: PM
As per gas laws
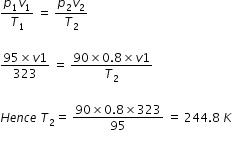
Answered by Ramandeep | 01 Nov, 2017, 11:26: AM
Concept Videos
ICSE 9 - Chemistry
Asked by zainaali39692 | 04 Dec, 2020, 08:53: AM
ICSE 9 - Chemistry
Asked by gup.navya2006 | 01 Dec, 2020, 09:28: AM
ICSE 9 - Chemistry
Asked by Vishusingh2020.2021 | 25 Sep, 2020, 10:09: PM
ICSE 9 - Chemistry
Asked by sudesghnapattanayak2017 | 19 May, 2020, 08:13: PM
ICSE 9 - Chemistry
Asked by abeshchakraborty6 | 23 Feb, 2020, 08:54: AM
ICSE 9 - Chemistry
Asked by dnlwalkers | 08 Jan, 2020, 09:57: AM
ICSE 9 - Chemistry
Asked by raichuratanvi | 14 Dec, 2019, 12:02: PM
ICSE 9 - Chemistry
Asked by merajanjum87 | 21 Nov, 2019, 09:53: PM
ICSE 9 - Chemistry
Asked by parvathimanjunath24 | 31 Oct, 2019, 09:34: PM
ICSE 9 - Chemistry
Asked by devbhaisha.tl | 21 Sep, 2019, 10:02: PM



