CBSE Class 11-science Answered
a compound which contains one atom of X, two atoms of Y for each three atoms of Z is made by mixing 5g X, 1.15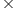 1023 atoms of Y and 0.03mol atoms of Z. if only 4.4g of the compound is produced in the process, calculate the atomic mass of Y given that atomic masses of X and Z are 60u and 80u respectively.
1023 atoms of Y and 0.03mol atoms of Z. if only 4.4g of the compound is produced in the process, calculate the atomic mass of Y given that atomic masses of X and Z are 60u and 80u respectively.
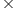 1023 atoms of Y and 0.03mol atoms of Z. if only 4.4g of the compound is produced in the process, calculate the atomic mass of Y given that atomic masses of X and Z are 60u and 80u respectively.
1023 atoms of Y and 0.03mol atoms of Z. if only 4.4g of the compound is produced in the process, calculate the atomic mass of Y given that atomic masses of X and Z are 60u and 80u respectively.
Asked by debaratib24 | 28 Jun, 2016, 21:17: PM
Answered by Arvind Diwale | 29 Jun, 2016, 12:36: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by rathodhamirbhai94 | 12 Jul, 2024, 21:34: PM
CBSE 11-science - Chemistry
Asked by ajjubhaikhgg851205khgg | 28 May, 2024, 13:30: PM
CBSE 11-science - Chemistry
Asked by anithaanu629940 | 21 May, 2024, 10:12: AM
CBSE 11-science - Chemistry
Asked by r84314179 | 10 May, 2024, 09:04: AM
CBSE 11-science - Chemistry
Asked by r84314179 | 06 May, 2024, 14:28: PM
CBSE 11-science - Chemistry
Asked by shreekrishnampatil | 27 Apr, 2024, 22:31: PM
CBSE 11-science - Chemistry
Asked by hm6561889 | 15 Apr, 2024, 07:45: AM
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 15:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 22:18: PM








