CBSE Class 12-science Answered
55Cs124(Z=55,A=124) has a half life of 30.8s.
(a) If we have 7.8 microgram initially, how many nuclei are presents? How many are present 2 min later?
(b) What is the activity at this time?
(c) After how much time will the activity drop to less than about 1 per second?
Asked by arjunsah797 | 16 May, 2022, 14:17: PM
Part (a)
Number of atoms in w gram = ( N / A ) × w
where N = 6.022 × 1023 is Avagadro number and A is atomic weight
Number of atoms in 7.8 microgram = ( 6.022 × 1023 / 124 ) × 7.8 × 10-6 = 3.788 × 1016
Number of nuclei at present, No = 3.788 × 1016
Number N of nuclei after 2 min is determined from radioactivity equation
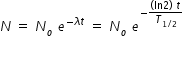
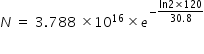
N = 2.544 × 1015
-----------------------------------------------
Part (b)
Activity after 2 mins is calculated from the following relation
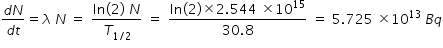
------------------------------------------------
Part (c)
Radioactivity is given as ( dN/dt ) = λ N
If (dN/dt ) = 1 Bq , then N = 1/λ = T1/2 / ln(2) ≈ 44
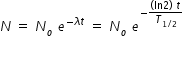
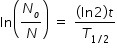
t = [ ln( No / N ) × T1/2 ] / (ln2)
after substituting values No = 2.544 × 1015 , N = 44 , T1/2 = 30.8 s , we get
t = 1408 s = 23 min 28 s
Answered by Thiyagarajan K | 16 May, 2022, 15:16: PM
Concept Videos
CBSE 12-science - Physics
Asked by prithviraj.chopra2011 | 14 Jul, 2024, 23:00: PM
CBSE 12-science - Physics
Asked by arjunsah797 | 16 May, 2022, 14:17: PM
CBSE 12-science - Physics
Asked by merinlijo_20002 | 17 Jun, 2020, 10:05: AM
CBSE 12-science - Physics
Asked by rajubarman | 01 Dec, 2019, 10:03: AM
CBSE 12-science - Physics
Asked by alanpeter9611 | 23 Feb, 2019, 19:47: PM
CBSE 12-science - Physics
Asked by alanpeter9611 | 22 Feb, 2019, 23:24: PM
CBSE 12-science - Physics
Asked by sd2021667 | 04 Dec, 2018, 16:46: PM
CBSE 12-science - Physics
Asked by silladech | 16 Nov, 2018, 11:14: AM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 09 Jul, 2014, 16:06: PM






 , find the number of nuclei present after 8 days?
, find the number of nuclei present after 8 days?