CBSE Class 11-science Answered
28.32 li!tres of chlorine w€re liberated at nornal
conditioDs of temperature and pressure. Calc.lllate
the \olume ofthe gas at l2oCaod 780 mm pressure.
Asked by ashutoshkumarchoubey845 | 21 Jul, 2021, 08:56: AM
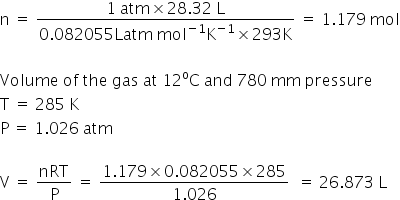
No. of moles at NTP, n= PV/RT
R= 0.082055 L atm mol-1 K-1
V= 28.32 L
T = 293 K
P = 1 atm
R= 0.082055 L atm mol-1 K-1
V= 28.32 L
T = 293 K
P = 1 atm
Answered by Ramandeep | 21 Jul, 2021, 12:25: PM
Application Videos
Concept Videos
CBSE 11-science - Chemistry
Asked by saranyachakraborty2007 | 25 Apr, 2024, 05:23: AM
CBSE 11-science - Chemistry
Asked by rhythmdraco42 | 22 Apr, 2024, 10:43: PM
CBSE 11-science - Chemistry
Asked by hm6561889 | 15 Apr, 2024, 07:45: AM
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by ansh.skulkarni1158 | 07 Apr, 2024, 11:03: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by josephineanto1960 | 28 Mar, 2024, 12:50: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM













