CBSE Class 10 Answered
1.with the help of an activity explain how metal reacts with acid draw diagram?
2. define organic acids and name the source of the following acid,lactic acid, tartaric acid,malic and acetic acids..?
Asked by bhavikabhatia1125 | 03 Jul, 2021, 12:16: PM
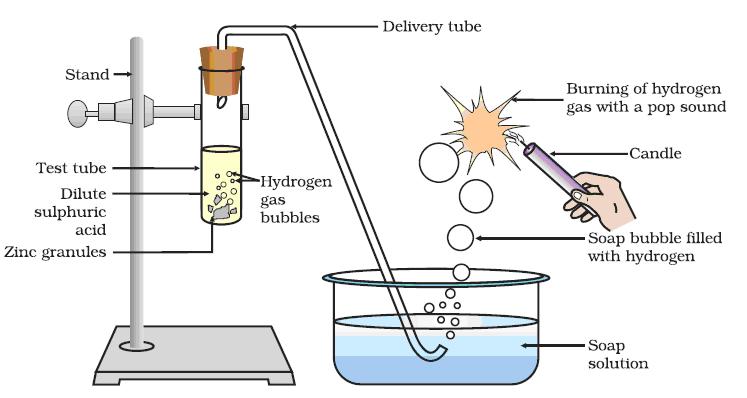
Acid reacts with metal to form hydrogen gas and salt.
Required Materials : 1. Test tube 2. Delivery tube 3. Glass trough 4. candle 5. Soap water 6. Dil. HCl 7. Zinc granules.
Procedure :
-Set the apparatus as shown in figure.-Take about 10 ml of dilute HCl in a test tube and add a few zinc granules to it.
-We will observe the formation of gas bubbles on the surface of zinc granules.-Pass the gas being evolved through the soap water.
-Gas filled bubbles are formed in the soap solution which rise into the air.-Bring a burning candle near the gas filled bubbles.-The gas present in a soap bubble burns with a ‘pop’ sound.
Result:
-Only ‘hydrogen’ gas burns making a ‘pop’ sound.-So we will notice that gas evolved is H2
Result:
-Only ‘hydrogen’ gas burns making a ‘pop’ sound.-So we will notice that gas evolved is H2
Answered by Ravi | 04 Jul, 2021, 21:05: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by toppercontentteam | 18 Jun, 2024, 13:09: PM
CBSE 10 - Chemistry
Asked by amanazeez6 | 14 Dec, 2023, 13:10: PM
CBSE 10 - Chemistry
Asked by ssyedbasha49 | 16 Jan, 2023, 14:17: PM
CBSE 10 - Chemistry
Asked by murugan3 | 25 Dec, 2022, 06:12: AM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 23 Nov, 2021, 02:40: AM
CBSE 10 - Chemistry
Asked by oyaulakh | 15 Sep, 2021, 06:55: AM
CBSE 10 - Chemistry
Asked by bhavikabhatia1125 | 03 Jul, 2021, 12:16: PM
CBSE 10 - Chemistry
Asked by bhavikabhatia1125 | 02 Jul, 2021, 22:14: PM
CBSE 10 - Chemistry
Asked by vpaishwarya061 | 14 Jun, 2021, 22:34: PM
CBSE 10 - Chemistry
Asked by nityashreebs | 18 Mar, 2021, 19:36: PM