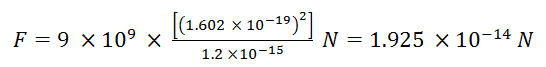CBSE Class 12-science Answered
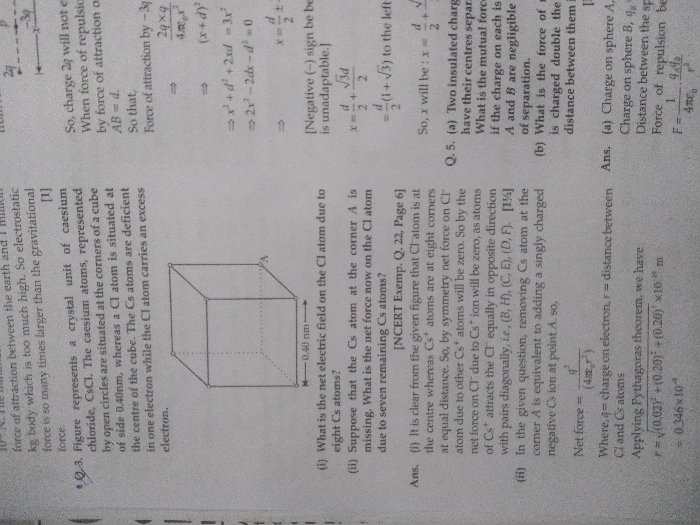
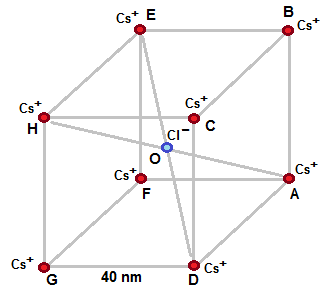
Figure shows the CsCl lattice that is made in a cube of side 40 nm . Positively charged Cs ions ( deficient in one electron )
are at corners of cube and negatively charged chlorine ion ( excess of one electron ) is at centre O of cube.
Attractive electrostatic force between Cl ion and Cs ion at A is nullified by attractive electrostatic force between Cl ion and Cs ion at H .
Similarly , Attractive electrostatic force between Cl ion and Cs ion at D is nullified by attractive electrostatic force between Cl ion and Cs ion at E .
Attractive electrostatic force between Cl ion and Cs ion at G is nullified by attractive electrostatic force between Cl ion and Cs ion at B
Attractive electrostatic force between Cl ion and Cs ion at F is nullified by attractive electrostatic force between Cl ion and Cs ion at C .
Hence net electrostatic force on Cl ion is zero .
----------------------------------------------------------------------------
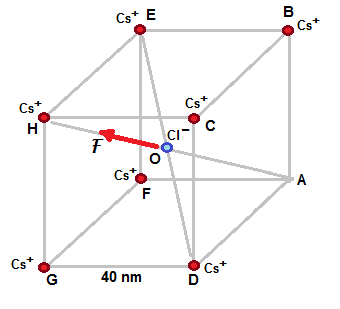
If Cs ion at A is removed , then attractive electrostatic force between Cl ion and Cs ion at H as shown in above fifure is the only force that is unbalanced .
Other forces between Cl ion and Cs ion at oter corners are nullified as explained in previous part.
Hence net force on Cl ion is given by
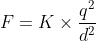
where K = 1/(4πεo ) = 9 × 109 N m2 C-2 is Coulomb's constant , q is magnitude of charge on each ion
and d is distance between Cl ion and Cs ion .
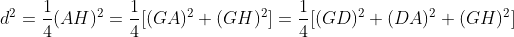
d2 = (3/4) [ 40 × 10-9 ]2 = 1.2 × 10-15 (nm)2
Hence net force on Cl ion is given by