CBSE Class 11-science Answered
12.5g of fumming H2SO4 (labeled 112+) is mixed with 100 litre water calculate mole concentration of h+ in resultant solution
Asked by puneetgarg2555 | 22 May, 2018, 07:10: AM
Given :
mass = 12.5 gm
% of H2SO4= 112%=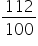
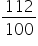
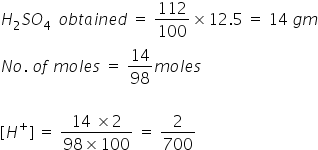
Answered by Ramandeep | 25 May, 2018, 06:31: PM
Application Videos
Concept Videos
CBSE 11-science - Chemistry
Asked by gklakshmi701 | 27 Apr, 2024, 09:36: AM
CBSE 11-science - Chemistry
Asked by saranyachakraborty2007 | 25 Apr, 2024, 05:23: AM
CBSE 11-science - Chemistry
Asked by rhythmdraco42 | 22 Apr, 2024, 10:43: PM
CBSE 11-science - Chemistry
Asked by hm6561889 | 15 Apr, 2024, 07:45: AM
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by ansh.skulkarni1158 | 07 Apr, 2024, 11:03: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by josephineanto1960 | 28 Mar, 2024, 12:50: PM













